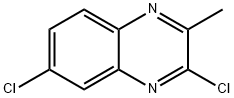
3,6-Dichloro-2-Methylquinoxaline synthesis
- Product Name:3,6-Dichloro-2-Methylquinoxaline
- CAS Number:76672-21-4
- Molecular formula:C9H6Cl2N2
- Molecular Weight:213.06
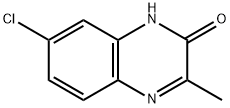
17796-60-0
3 suppliers
inquiry

76672-21-4
5 suppliers
inquiry
Yield:76672-21-4 86%
Reaction Conditions:
with trichlorophosphate at 120; for 5 h;
Steps:
4.1.3 General procedure for 2-chloro-3-methyl substituted quinoxaline (2)
General procedure: Compound 1a (28.1mmol, 5.0g) was dissolved in POCl3 (25mL) at 0°C, the resulting solution was allowed to reflux at 120°C for 5h. After completion of reaction, the reaction was quenched with cooled ammonia, which was further extracted with ethyl acetate (EtOAc, 2×15mL). The organic phase was separated and washed with brine, dried over anhydrous MgSO4, filtered, and concentrated in vacuum to afford the crude product, which was purified by silica gel flas chromatography (PE: EA=10:1, v:v) to afford 5.0g of 2a as an off-white solid, yield 90%.
References:
Xia, Qiao-Hong;Hu, Wei;Li, Chen;Wu, Ji-Feng;Yang, Liang;Han, Xue-Mei;Shen, Yue-Mao;Li, Zhi-Yu;Li, Xun [European Journal of Medicinal Chemistry,2016,vol. 124,p. 311 - 325] Location in patent:supporting information

17796-60-0
3 suppliers
inquiry

76672-27-0
3 suppliers
inquiry

76672-21-4
5 suppliers
inquiry

76672-20-3
0 suppliers
inquiry

617-35-6
522 suppliers
$5.00/10g

95-83-0
288 suppliers
$14.00/5g

76672-21-4
5 suppliers
inquiry

76672-20-3
0 suppliers
inquiry

95-83-0
288 suppliers
$14.00/5g

76672-21-4
5 suppliers
inquiry
