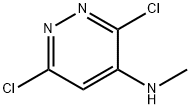
3,6-DICHLORO-N-METHYL-4-PYRIDAZINAMINE synthesis
- Product Name:3,6-DICHLORO-N-METHYL-4-PYRIDAZINAMINE
- CAS Number:17645-06-6
- Molecular formula:C5H5Cl2N3
- Molecular Weight:178.02
Yield:17645-06-6 93.7%
Reaction Conditions:
in tetrahydrofuran at 50; for 2 h;Sealed tube;
Steps:
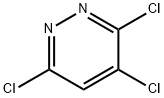
3,6-dichloro-N-methylpyridazin-4-amine (1b)
3,4,6-Trichloropyridazine (500mg, 2.73 mmol, Eq: 1) and methanamine (2 M solutionin THF, 10.9 ml, 21.8 mmol, Eq: 8) were combined in a glass tube resulting in a light yellow suspension. The tube was sealed and heated to 50 °C for two hours. The mixture was diluted with water and ethyl acetate and then extracted 4 times with ethyl acetate. The combined organic layers were washed once with brine, dried with sodium sulfate, filtered and concentrated in vacuo. 3,6-Dichloro-N-methylpyridazin-4-amine was obtained as alight yellow powder (454.6 mg, 93.7%) and was used without further purification. LC-HRMS: m/z=177.9938 [(M+H)+ calcd for C5H5Cl2N3: 177.9933; Diff 0.5 mDa]. 1H NMR(CHLOROFORM-d, 600MHz): (ppm) 6.53 (s, 1H), 5.20 (br. s., 1H), 2.97 (d, J=5.1 Hz, 3H). 13C NMR (CHLOROFORM-d, 151MHz): (ppm) 155.7, 144.7, 144.1, 104.9, 29.2.
References:
Stoll, Theodor;Alker, André;Kolczewski, Sabine;Menzi, Andrea;Revil-Baudard, Vincent [Tetrahedron Letters,2015,vol. 56,# 6,p. 772 - 774]