
3,6-Dihydro-2H-1,2-oxazine hydrochloride synthesis
- Product Name:3,6-Dihydro-2H-1,2-oxazine hydrochloride
- CAS Number:872-41-3
- Molecular formula:C4H8ClNO
- Molecular Weight:121.5654
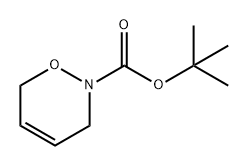
147462-15-5
0 suppliers
inquiry

872-41-3
7 suppliers
inquiry
Yield:872-41-3 72%
Reaction Conditions:
with hydrogenchloride in methanol;water at 0 - 20; for 24 h;
Steps:
Deprotection of Boc-Protected Hydroxylamines; General Procedure GP3
General procedure: In a round-bottom flask, the Boc-protected hydroxylamine (1.0 equiv) was dissolved in MeOH and HCl (37% aq, 1 mL/mmol) was added dropwise at 0 °C. The reaction solution was stirred at r.t. until complete consumption of the starting material was detected by TLC, and subsequently cooled to 5 °C and the pH was adjusted to pH 9 by the addition of NaOH (20% aq). The organic solvent was removed under reduced pressure and the aqueous residue was extracted three times with EtOAc. The combined organic layer was dried over MgSO4, filtered and the solvent was removed under reduced pressure to obtain the product.
References:
F?hrmann, Jan;Hermann, Ludmila;Hilt, Gerhard [Synthesis,2022,vol. 54,# 8,p. 2005 - 2018]