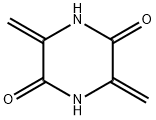
3,6-Methylene-2,5-piperazinedione synthesis
- Product Name:3,6-Methylene-2,5-piperazinedione
- CAS Number:15996-22-2
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12
![(3R,6R)-3,6-Bis[(aminooxy)methyl]-2,5-piperazinedione](/CAS/GIF/16337-02-3.gif)
16337-02-3
40 suppliers
$120.00/10mg

15996-22-2
34 suppliers
inquiry
Yield:15996-22-2 80%
Reaction Conditions:
with sodium hydroxide in water at -5 - 30; for 20 h;
Steps:
3,6-Dimethylidenepiperazine-2,5-dione (1b)
A stirring solution of compound 1a (10 g, 0.05 mol) in water (250 ml)was cooled to -5-0°C on an ice bath. A solution of NaOH (160 mg, 0.004 mol) in water (50 ml) was added dropwiseat -5 -0°C. The reaction mixture was warmed up to 25-30°C,and product starts to precipitate. The reaction mixture was stirred at 25-30°C for 20 h. Pure product was isolated by filtration, washed with cold ethanol (5×3 ml), and driedunder reduced pressure at 50°C to afford product 1b. Yield 5.5 g (80%), white solid, mp 380-390°C (decomp.)(mp >300°C 30). IR spectrum, ν, cm-1: 1687 (C=O), 3182(NH). 1H NMR spectrum (DMSO-d6), δ, ppm: 4.91 (2H, s,CH2); 5.26 (2H, s, CH2); 10.89 (2H, s, 2NH). 13C NMRspectrum (DMSO-d6), δ, ppm: 100.9 (2C); 135.1 (2C);156.6 (2C). Mass spectrum, m/z (Irel,%): 137 [M-H]- (100).Found, %: C 52.15; H 4.41; N 20.29. C6H6N2O2.Calculated, %: C 52.17; H 4.38; N 20.28.
References:
Awasthi, Arun Kumar;Kumar, Brijesh;Aga, Mushtaq A;Tripathi, Punit;Reddy, Cirandur Suresh;Kumar, Pramod [Chemistry of Heterocyclic Compounds,2017,vol. 53,# 11,p. 1248 - 1253][Khim. Geterotsikl. Soedin.,2017,vol. 53,# 11,p. 1248 - 1253,6]
![2,5-Piperazinedione, 3,6-bis[(acetyloxy)methyl]-](/CAS/20200515/GIF/5249-15-0.gif)
5249-15-0
1 suppliers
inquiry

15996-22-2
34 suppliers
inquiry
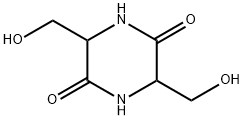
5625-41-2
35 suppliers
inquiry

15996-22-2
34 suppliers
inquiry