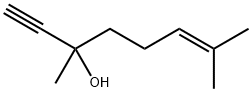
3,7-dimethyloct-6-en-1-yn-3-ol synthesis
- Product Name:3,7-dimethyloct-6-en-1-yn-3-ol
- CAS Number:29171-20-8
- Molecular formula:C10H16O
- Molecular Weight:152.23
The specific process is as follows: open the valves of ammonia and acetylene gas cylinders, respectively, add ammonia gas and acetylene to the mixed gas cabinet, and adjust the ratio of the two gases so that the content of acetylene in the mixed gas cabinet is about 20–25%. The gas mixture is compressed to 2.0–2.5 MPa by a compressor, liquefied in a high-position cooling tower and enters the reaction tower through a bottom discharge pipe, and a methylheptene solution containing potassium hydroxide is introduced into the reaction tower through a metering pump. The reaction temperature is 35–40°C, the reaction pressure is 2.0–2.5 MPa and the reaction time is 2–3 h. The reaction liquid enters the flash tank through the discharge tube of the reaction tower, and the flashing temperature is 50–70°C. Excess acetylene and ammonia are flashed and recycled back to the mixing gas cabinet, and the reaction product overflows from the bottom of the flash tank to the storage tank. By distillation under reduced pressure, dehydrolinalool having a mass fraction of more than 98% can be obtained and the yield can reach 92–94%.
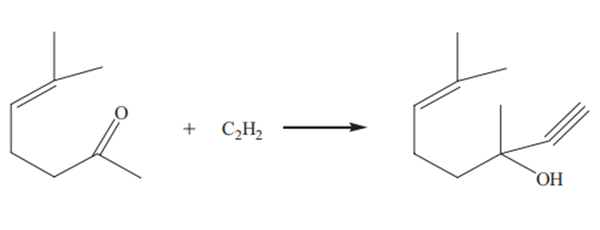
Yield: 99.1%
Reaction Conditions:
with sodium methylate in methanol at 0 - 5; for 0.5 h;Inert atmosphere;
Steps:
5 Example 1. Preparation of methylbutynol
General procedure: In a 10 L three-necked flask, 6 L of anhydrous methanol was added, and the mixture was shielded with nitrogen. Under cooling with ice water, 550 g of sodium metal (small) was slowly added.After all the sodium metal was dissolved, 1.5 L of acetone was added, and the mixture was uniformly stirred and cooled to below 0 °C. Slowly pass acetylene, The flow rate of the acetylene is controlled so that the reaction temperature is 0 to 5 °C. When the reaction is no longer exothermic, the reaction is complete. Then, a small amount of acetylene was introduced, and the closed micro-positive pressure (nitrogen balloon was not changed) was reacted for 0.5 hour. In another 20 L three-necked flask, 2.5 kg of ammonium chloride and 3 L of methanol were added.Under ice cooling, the reaction solution was poured into an ammonium chloride system to carry out a neutralization reaction. The neutralization reaction temperature was controlled to be less than 20 °C. After the neutralization is completed, it is filtered. The filtrate was first distilled with methanol, methanol was applied, and then the product methylbutynol was distilled under reduced pressure to obtain 1.69 kg. The product purity was 99.5%, and the yield was 99.1%.
References:
Panjin Ge Linkaimo Technology Co., Ltd.;Gong Ningrui CN108863717, 2018, A Location in patent:Paragraph 0036; 0037; 0038; 0043; 0044; 0045; 0046
110-93-0
321 suppliers
$8.00/25g
4301-14-8
127 suppliers
$55.20/100ml

29171-20-8
62 suppliers
$16.00/5g

110-93-0
321 suppliers
$8.00/25g

74-86-2
78 suppliers
inquiry

29171-20-8
62 suppliers
$16.00/5g
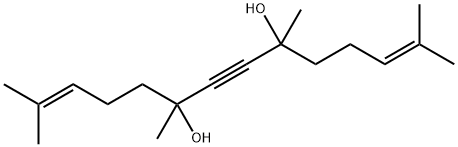
62179-75-3
1 suppliers
inquiry
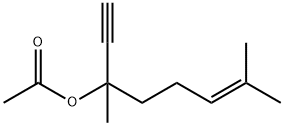
29171-21-9
7 suppliers
inquiry
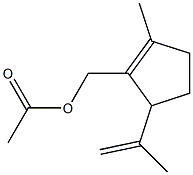
80113-88-8
0 suppliers
inquiry

29171-20-8
62 suppliers
$16.00/5g
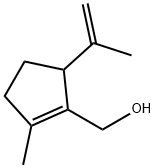
60168-94-7
0 suppliers
inquiry

110-93-0
321 suppliers
$8.00/25g

1066-26-8
99 suppliers
$48.00/50g

29171-20-8
62 suppliers
$16.00/5g