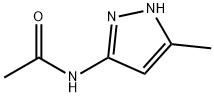
3-Acetamido-5-methylpyrazole synthesis
- Product Name:3-Acetamido-5-methylpyrazole
- CAS Number:83725-05-7
- Molecular formula:C6H9N3O
- Molecular Weight:139.16
31230-17-8
348 suppliers
$5.00/5g

108-24-7
5 suppliers
$14.00/250ML

83725-05-7
52 suppliers
$24.00/100mg
Yield:83725-05-7 62%
Reaction Conditions:
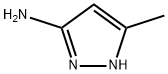
Stage #1: 3-methyl-5-aminopyrazolewith sodium hydrogencarbonate in water at 20; for 0.5 h;
Stage #2: acetic anhydride in water at 100; for 3 h;
Steps:
1.1 Step 1: Synthesis of N-(5-methyl-1H-pyrazol-3-yl)acetamide
5-methyl-1H-pyrazol-3-amine (300 g, 3.09 mol, 1.0 eq) was weighted into a 5 L round bottom flask, dissolved by adding water (2800 mL) with mechanical stirring at room temperature. Sodium bicarbonate (780 g, 9.28 mol, 3 eq) was added in portions, following by stirring for another 30 min after the addition. Then, acetic anhydride (592 ml, 6.2 mol, 2 eq) was added dropwise and slowly to the reaction system over about 1 h at controlled drop rate. At this point, a large amount of foamy white solid was produced. The temperature was raised to 100° C. and the reaction system was stirred for 2 h. The solid was gradually dissolved, and the system became clarification. Then heating was stopped, and the system was cooled to room temperature. After stirring overnight, a large amount of white crystalline solid precipitated. Another batch of 5-methyl-1H-pyrazol-3-amine (300 g) was fed in a parallel reaction. After the reactions were completed, the two batches were combined and filtered, and the solid was washed with water (500 mL*2) and dried to give a white solid (554 g, yield 62%). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 10.32 (s, 1H), 6.21 (s, 1H), 2.18 (s, 3H), 1.97 (s, 3H).
References:
US2019/40064,2019,A1 Location in patent:Paragraph 0155; 0156

31230-17-8
348 suppliers
$5.00/5g

83725-05-7
52 suppliers
$24.00/100mg

31230-17-8
348 suppliers
$5.00/5g

75-36-5
560 suppliers
$17.92/100G

83725-05-7
52 suppliers
$24.00/100mg