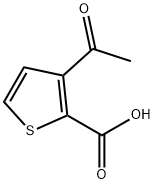
3-ACETYLTHIOPHENE-2-CARBOXYLIC ACID synthesis
- Product Name:3-ACETYLTHIOPHENE-2-CARBOXYLIC ACID
- CAS Number:13657-90-4
- Molecular formula:C7H6O3S
- Molecular Weight:170.19
Yield:13657-90-4 41%
Reaction Conditions:
with 2,3,6,7-tetramethoxy-9(10H)-anthracenone;caesium carbonate in dimethyl sulfoxide at 25; under 2280.15 Torr; for 18 h;Irradiation;Sealed tube;regioselective reaction;
Steps:
General Procedure for the Carboxylation of Hetero(arenes) and Styrenes- GP2a
General procedure: To a dry flat-bottomed crimp vial (5 mL) equipped with stirring bar, was added the arene (if solid)(0.1 mmol) and 2,3,6,7-tetramethoxyanthracen-9(10H)-one (6.3 mg, 0.02 mmol, 20 mol %). Cs2CO3 (98 mg,3 equiv.) was quickly added and the vial was sealed with a Supelco aluminium crimp seal with septum(PTFE/butyl). The vial was then evacuated and refilled with CO2 (5×) via syringe needle. The reactionmixture was dissolved in DMSO (1 mL, dry and degassed by bubbling with N2) and the arene (0.1 mmol) (ifliquid) was added via syringe. The vial was sealed with two layers of Parafilm and then had gaseous CO2added via a Luer Lock Monoject (20 ccm) syringe, into the head space. The vial was then irradiated fromthe bottom side with blue LED light and a constant reaction temperature (25°C) was maintained byemploying a water-cooling circuit connected to a thermostat. After 18 hrs of reaction time the pressure wasreleased. For product isolation, the reaction mixtures of 4 reactions run in parallel were combined andtransferred with water and Et2O into a separating funnel. The ether layer was extracted with water (3×) andthe combined aqueous layers were acidified with aq. HCl (2M) to adjust to an acidic pH. The aqueous layerwas extracted with EtOAc (3×) and the combined EtOAc layers were dried over Na2SO4, filtered andconcentrated in vacuo. The crude material was purified by silica flash column chromatography usingmixtures of hexanes and ethyl acetate with 0.5% HOAc (v/v) as eluents.
References:
Bergonzini, Giulia;Brandt, Peter;Fricke, Florian;Johansson, Magnus J.;K?nig, Burkhard;Schmalzbauer, Matthias;Svejstrup, Thomas D. [Chem,2020,vol. 6,# 10,p. 2658 - 2672] Location in patent:supporting information
43039-99-2
5 suppliers
inquiry

13657-90-4
14 suppliers
inquiry
1468-83-3
300 suppliers
$5.00/1g

13657-90-4
14 suppliers
inquiry