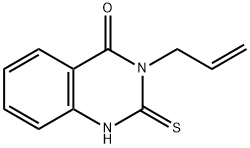
3-ALLYL-2-MERCAPTO-3H-QUINAZOLIN-4-ONE synthesis
- Product Name:3-ALLYL-2-MERCAPTO-3H-QUINAZOLIN-4-ONE
- CAS Number:21263-59-2
- Molecular formula:C11H10N2OS
- Molecular Weight:218.27

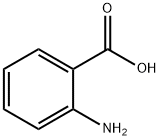
10341-86-3
10 suppliers
$89.00/20mg

21263-59-2
19 suppliers
$28.60/25mg
Yield:21263-59-2 92%
Reaction Conditions:
with carbon disulfide;potassium hydroxide in ethanol; for 3 h;Reflux;
Steps:
General Experimental Procedure for 2-thioxo- 2,3-dihydroquinazolin-4(1H)-one derivatives
General procedure: A solution of isatoic anhydride (1 mmol) and appropriate amine (1 mmol) in 10 ml EtOH was stirred at reflux for 3 h. Then, a mix- ture of carbon disulfide (2 mmol) and KOH (1 mmol) was added to the reaction mixture and stirring continued under the same condi- tions for 3 h. The reaction mixture was cooled to room tempera- ture and poured into 20 ml water. The precipitate was collected by filtration, washed with water, and recrystallized using EtOH to give desirable product as colorless crystals.
References:
Adibi, Hossein;Asgari, Mohammad Sadegh;Attarroshan, Mahshid;Farid, Sara Moghadam;Hamedifar, Haleh;Hosseini, Samanesadat;Iraji, Aida;Kabiri, Maryam;Khoshneviszadeh, Mehdi;Larijani, Bagher;Mahdavi, Mohammad;Moayedi, Seyedeh Sara;Moazzam, Ali;Pirhadi, Somayeh;Sakhteman, AmirHossein;Sepehri, Nima [Journal of Molecular Structure,2022,vol. 1253,art. no. 132283]

75-15-0
236 suppliers
$40.00/100g

4943-82-2
18 suppliers
$36.00/100mg

21263-59-2
19 suppliers
$28.60/25mg
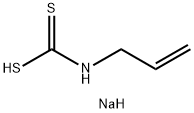
40135-33-9
0 suppliers
inquiry
134-20-3
576 suppliers
$18.00/25mL

21263-59-2
19 suppliers
$28.60/25mg

75-15-0
236 suppliers
$40.00/100g
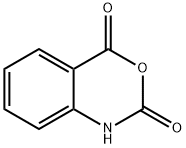
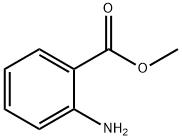
118-48-9
412 suppliers
$5.00/5G

107-11-9
1 suppliers
$31.80/25ml

21263-59-2
19 suppliers
$28.60/25mg