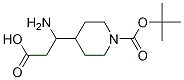
3-AMino-3-(1-Boc-4-piperidyl)propanoic Acid synthesis
- Product Name:3-AMino-3-(1-Boc-4-piperidyl)propanoic Acid
- CAS Number:372144-02-0
- Molecular formula:C13H24N2O4
- Molecular Weight:272.34

141-82-2
760 suppliers
$5.00/25g
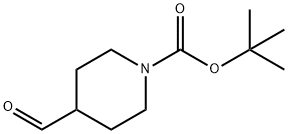
137076-22-3
365 suppliers
$5.00/1g

372144-02-0
23 suppliers
$169.00/250mg
Yield:372144-02-0 105 g
Reaction Conditions:
with ammonium acetate in ethanol at 100;
Steps:
1.3 Example 1 Preparation of N-BOC-4- (1-amino-3-hydroxypropyl) piperidine, the specific steps are as follows
(3)205 g of compound 3, 1223 g of malonic acid, 185 g of ammonium acetate was dissolved in 500 ml of ethanol and heated at 100 ° CThe reaction was complete, the reaction was complete, the system was cooled, filtered and the cake was dried to give 105 g of compound 4 (white solid) in a yield of 39%
References:
CN105272905,2016,A Location in patent:Paragraph 0023; 0032