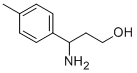
3-AMINO-3-P-TOLYL-PROPAN-1-OL synthesis
- Product Name:3-AMINO-3-P-TOLYL-PROPAN-1-OL
- CAS Number:68208-23-1
- Molecular formula:C10H15NO
- Molecular Weight:165.23
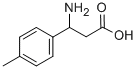
68208-18-4
128 suppliers
$45.00/50mg

68208-23-1
37 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 5.5 h;Inert atmosphere;Reflux;
Steps:
3-(Benzoyloxy)-N-(2-(tert-butoxy)-2-oxoethyl)-1-(4-chlorophenyl)-N,N-dimethylpropan-1-aminiumbromide (1j)
General procedure: (Step 2) 22 (613 mg, 3.07 mmol) was added slowly to a suspension of LiAlH4 (0.18 g, 4.7 mmol)in THF (10 mL) at 0 °C under an Ar atmosphere. The mixture was stirred for 30 min at 0 °C and refluxed for5 h. The resulting mixture was cooled to 0 °C, diluted with Et2O, and quenched with H2O (0.18 mL). The mixture was treated with 15 wt.% NaOH in H2O (0.18 mL) followed by H2O (0.54 mL) and stirred for over30 min at room temperature. The mixture was filtered through a pad of Celite and the filtrate was evaporated.The residue was recrystallized from toluene to obtain 3-amino-3-(4-chlorophenyl)propan-1-ol (23) (291 mg,51% yield) as a white solid
References:
Baba, Souya;Hirano, Kazuki;Tayama, Eiji [Tetrahedron,2020] Location in patent:supporting information
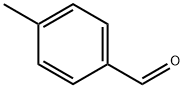
104-87-0
510 suppliers
$6.00/25g

68208-23-1
37 suppliers
$45.00/50mg