Yield:-
Reaction Conditions:
Stage #1:DIV01389 with trifluoroacetic acid at 0 - 60; for 2 h;
Stage #2: with sodium hydrogencarbonate in water at 20;Product distribution / selectivity;
Steps:
1.06; 2.09; 3.10; 4.07; 5.03; 6.07; 7.03; 8.10; 9.09; 10.03; 11.03; 12.03; 13.10; 14.10; 15.10; 16.08; 17.07; 18.06; 19.03; 20.07; 21.10; 22.10; 23.09; 24.08
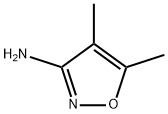
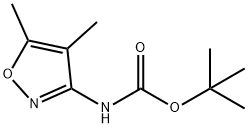
STEP06: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluroacetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP09: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEPlO: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluoroacetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP07: Synthesis of 4, 5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP03: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP07: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP03: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifiuro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEPlO: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP09: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP03: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP03: Synthesis of 4.5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP03: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine;.(4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEPlO: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEPlO: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEPlO: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP08: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; H3CN CH3 (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP07: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; 3(4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP06: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.; STEP03: Synthesis of 4,5-dimethyl-isoxazol-3-yl -amine.; (4, 5-dimethyl-isoxazol-3-yl) carbamic acid tert-butyl ester (22 gm, 0.1036 mol) was o portion wise added to the trifluro acetic acid (22 ml 0.3108 mol) at 0 C. After the o completion of the addition, the reaction mixture was warmed to 60 C and stirred it for 2 hrs. The reaction mixture was cooled to room temperature and basified with a saturated solution of sodium bicarbonate. The product was extracted with methylene dichloride (100 ml x2). The organic layer was washed with water and brine solution. Finally organic layer was dried over sodium sulphate and evaporated under vacuum to give 9 gm of 4, 5- dimethyl-isoxazol-3-ylamine as yellow solid.
References:
TORRENT PHARMACEUTICALS LTD WO2007/100295, 2007, A1 Location in patent:Page/Page column 35-36; 46-47; 57; 67-68; 75; 85; 93; 104-105; 115
FullText