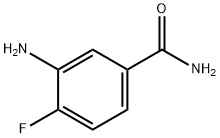
3-amino-4-fluorobenzamide synthesis
- Product Name:3-amino-4-fluorobenzamide
- CAS Number:943743-25-7
- Molecular formula:C7H7FN2O
- Molecular Weight:154.14
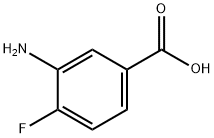
2365-85-7
192 suppliers
$9.00/1g

943743-25-7
23 suppliers
$60.00/100mg
Yield:943743-25-7 61%
Reaction Conditions:
with ammonium chloride;benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 0 - 20; for 12 h;
Steps:
11.1 3-amino-4-fluorobenzamide (1):
3-amino-4-fluorobenzamide (1): To a solution of 3-amino-4-fluorobenzoic acid (1.0 g, 6.4 mmol) in dimethylformamide (5.0 mL), l-Ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride. HC1 (1.2 g, 7.7 mmol), ammonium chloride (1.4 g, 26.9 mmol) and hydroxybenzotriazole (1.1 g, 8.3 mmol) were added at 0 °C. The resulting mixture was stirred at room temperature for 12 h. After completion of the reaction, ice-cold water was added to the reaction mixture and the aqueous layer was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduce pressure to afford 1 (600 mg, 61% ) as an orange-red solid. 1H NMR (400 MHz, DMSO-d6): δ 5.24 (brs, 2H), 6.97 (m, 2H), 7.13 (brs, 1H), 7.26 (d, J = 7.8 Hz, 1H), 7.74 (brs, 1H).
References:
WO2014/149164,2014,A1 Location in patent:Paragraph 00634