
3-aMino-5-broMo-4-chloropyridine synthesis
- Product Name:3-aMino-5-broMo-4-chloropyridine
- CAS Number:89283-92-1
- Molecular formula:C5H4BrClN2
- Molecular Weight:207.46
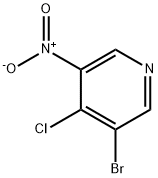
31872-63-6
177 suppliers
inquiry

89283-92-1
66 suppliers
$25.00/250mg
Yield: 72%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 80; for 2 h;
Steps:
1.c 5-Bromo-4-chloropyridin-3-amine
To a solution of 3-bromo-4-chloro-5-nitropyridine (9.50 g, 40.01 mmol) in EtOH (500 mL) was added NH4CI (13.27 g, 248 mmol), water (50 ml), and Fe (22.39 g, 401 mmol) at room temperature. The resulting mixture was stirred for 2 h at 80°C. Whenthe reaction was done, the solids were filtered off. The resulting mixture was concentrated under reduced pressure and the residue was purified by flash chromatography eluting with 0% to 60% EtOAc in petrol ether to yield 5-bromo-4- chloropyridin-3-amine as a yellow oil (5.94 g, 72%). MS: m/z = 209.2 [M+H].
References:
MERCK PATENT GMBH;TANZER, Eva-Maria;SCHIEMANN, Kai;KLEIN, Markus WO2019/25099, 2019, A1 Location in patent:Page/Page column 142