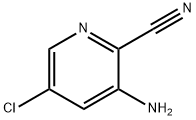
3-amino-5-chloropicolinonitrile synthesis
- Product Name:3-amino-5-chloropicolinonitrile
- CAS Number:408538-29-4
- Molecular formula:C6H4ClN3
- Molecular Weight:153.57
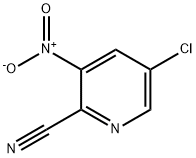
181123-11-5
172 suppliers
inquiry

408538-29-4
31 suppliers
inquiry
Yield:408538-29-4 96 %
Reaction Conditions:
with iron;acetic acid at 0 - 20;
Steps:
3 Synthesis of 3-amino-5-chloropicolinonitrile (23):
To a solution of 5-chloro-3- nitropicolinonitrile (22, 50 g, 0.273 mol) in acetic acid (250 mL) cooled to 0 °C, iron power (76.5 g, 1.36 mol) was added slowly in portions. The reaction was then stirred for 2 h when TLC showed consumption of starting material. The reaction mixture was then filtered over a bed of celite and celite bed was washed with methanol. The combined mother liquor was evaporated under reduced pressure and crude was dissolved in ethyl acetate followed by washing with sodium carbonate solution. The organic layer was washed with brine, dried over anhydrous sodium sulfate and solvent was removed under reduced pressure to afford 3- amino-5-chloropicolinonitrile (26, 40.0 g, 96%) as off white solid.
References:
WO2022/187178,2022,A1 Location in patent:Page/Page column 58-59

181123-11-5
172 suppliers
inquiry

408538-29-4
31 suppliers
inquiry

27330-34-3
39 suppliers
$101.00/100mg
90902-83-3
194 suppliers
$10.00/1g

408538-29-4
31 suppliers
inquiry
75806-86-9
160 suppliers
$14.00/1g

408538-29-4
31 suppliers
inquiry
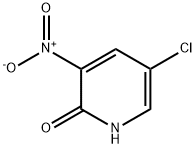
21427-61-2
177 suppliers
$6.00/1g

408538-29-4
31 suppliers
inquiry