
3-AMINO-6-METHOXYPYRAZINE-2-CARBOXYLIC ACID synthesis
- Product Name:3-AMINO-6-METHOXYPYRAZINE-2-CARBOXYLIC ACID
- CAS Number:16312-52-0
- Molecular formula:C6H7N3O3
- Molecular Weight:169.14
938439-10-2
0 suppliers
inquiry

16312-52-0
11 suppliers
inquiry
Yield:16312-52-0 82%
Reaction Conditions:
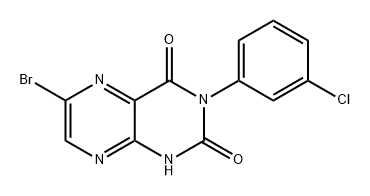
Stage #1: 6-bromo-3-(3-chlorophenyl)pteridine-2,4(1H,3H)-dionewith sodium methylate in methanol at 130; for 20 h;
Stage #2: with sodium hydroxide in methanol; for 20 h;Heating / reflux;
Stage #3: with hydrogenchloride in water at 20; pH=2 - 3; for 1 h;
Steps:
26H
6-Bromo-3-(3-chlorophenyl)pteridine-2,4(/H;3H)-dione prepared in Example 251 (6.750 g, 19.10 mmol, 1 eq.) was mixed with a 30 % solution sodium methoxide (3.094 g, 57.27 mmol, 3 eq.) in 84 mL anhydrous methanol. The mixture was heated at 130 0C for 20 h. LC/MS showed that the starting material was consumed. The solvent was removed in vacuo. To the residue, was added an aqueous solution of sodium hydroxide (1.145 g, 28.64 mmol, 1.5 eq. in 150 mL water). The mixture was refluxed for 20 h until the reaction was complete. The reaction mixture was then allowed to reach room temperature and a trace amount of insoluble solid was filtered out. The filtrate was decolored with charcoal and then evaporated to half the volume. The resulting solution was neutralised with 4 N aqueous hydrochloride solution until pH 2-3. After standing at room temperature for 1 h, the solids formed were filtered, rinsed with cold water twice, and dried in vacuo. 2.65 g of solid (82 %) was obtained, which was used for methylation without further purification. 1H NMR (400 MHz, DMSO-*) δ (ppm) 3.83 (s, 3H), 8.10 (s, IH).
References:
WO2007/61360,2007,A2 Location in patent:Page/Page column 70