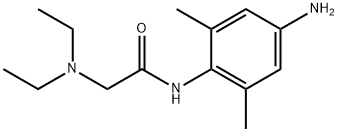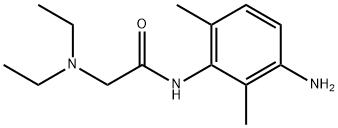
3-Amino Lidocaine synthesis
- Product Name:3-Amino Lidocaine
- CAS Number:39942-50-2
- Molecular formula:C14H23N3O
- Molecular Weight:249.35

39942-49-9
10 suppliers
inquiry

39942-50-2
14 suppliers
inquiry
Yield:39942-50-2 70%
Reaction Conditions:
with hydrogenchloride;iron in water at 5 - 30; for 1 h;
Steps:
9
Hydrochloric acid (6N, 210 mL) was stirred (mechanically) and cooled in an ice-water bath to 5° C, while powdered solid 3-nitro-lidocaine (27.0 g, 96.9 mmol) was added. To the resulting solution was added iron powder (25 g, 447 mmol), portionwise over 3-5 minutes. The rate of addition was such that the internal temperature did-not exceed 10° C. Gas evolution was observed (hydrogen). The reaction mixture was stirred an additional 30 minutes at 10-30° C then 30 minutes at 30°C using an external ice-water bath. Solid ice was added (400 g) followed by slow addition of solid NAOH until the reaction pH was 12 (internal temperature did not exceed 40° C during the neutralization process). The entire contents of the reaction flask were filtered through a bed of celite and the residual solids were washed with ethyl acetate (3 x 200 mL). The organic layer was separated, dried (MGSO4) and evaporated to give 19.0 g of a light yellow solid after recrystallization from ethyl ACETATE/HEXANE. Yield 70%.
References:
WO2005/25498,2005,A2 Location in patent:Page/Page column 19
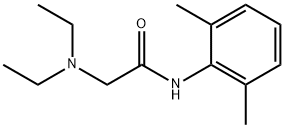
137-58-6
523 suppliers
$14.00/5g

39942-50-2
14 suppliers
inquiry