
3-AMINO-N-(4-METHOXYPHENYL)BENZAMIDE synthesis
- Product Name:3-AMINO-N-(4-METHOXYPHENYL)BENZAMIDE
- CAS Number:115175-19-4
- Molecular formula:C14H14N2O2
- Molecular Weight:242.27

101971-72-6
4 suppliers
inquiry

115175-19-4
9 suppliers
$60.00/50mg
Yield:115175-19-4 86%
Reaction Conditions:
with iron;ammonium chloride in methanol; for 7 h;Reflux;
Steps:
3.1.8. General Experimental Procedures of 4ii-8ii
General procedure: To a solution of 4i-8i (3.37 mmol) in methanol (20 mL) was added: ammonium chloride (doubleamounts of 4i-8i) and iron powder (6.03 mmol) and refluxed for 7 h. The reaction mixture wasevaporated to give an oily residue, which was added with water and extracted with dichloromethane(30 mL x 3). The combined organic phase was dried with anhydrous MgSO4, filtrated, and the filtratewas evaporated to yield a crude solid, which recrystallized with ethyl acetate and n-hexane to give apure white or pale yellow solid.
References:
Lee, Soo Hyun;Lee, Wonhwa;Bae, Jong-Sup;Ma, Eunsook [Molecules,2016,vol. 21,# 5,art. no. 676]
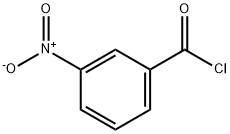
121-90-4
8 suppliers
$24.00/25g

115175-19-4
9 suppliers
$60.00/50mg
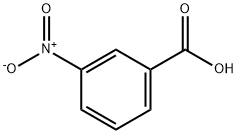
121-92-6
575 suppliers
$6.00/25g

115175-19-4
9 suppliers
$60.00/50mg