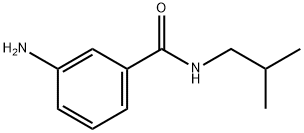
3-amino-N-isobutylbenzamide synthesis
- Product Name:3-amino-N-isobutylbenzamide
- CAS Number:81882-64-6
- Molecular formula:C11H16N2O
- Molecular Weight:192.26
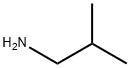
78-81-9
191 suppliers
$19.60/250ml
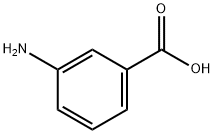
99-05-8
513 suppliers
$14.00/5g

81882-64-6
10 suppliers
$23.00/1g
Yield:81882-64-6 100%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20;
Steps:
2.9. General peptide coupling procedure for 3-amino-benzamides8b-k
General procedure: To a solution of the primary amine (7b-e) (1.3 eq) and therespective (substituted) 3-aminobenzoic acid (2.0 mmol, 1.0 eq) inDMF were added DIPEA (2.0 eq), N-(3-dimethylaminopropyl)-N0-ethylcarbodiimide hydrochloride (EDC*HCl) (2.0 eq) and 1-hydroxybenzotriazole (HOBt) (0.15e0.30 eq.) for the preparationof 8b,c,e-i,k. HATU or PyBrop was used as coupling reagent in thecase of products 8d and 8j respectively, which required purificationby column chromatography afterwards. The mixture was stirredovernight at RT. TLC showed full conversion of the starting materials.DMF was evaporated in vacuo where after the mixture wasdissolved in ethyl acetate, washed with NaHCO3 solution (aq.) onceand with brine twice. The organic layer was dried over MgSO4 andthe solvent evaporated in vacuo. The resulting compounds 8b-kwere used without further purification in the next reaction. Compound8a was obtained from a commercial source.3-amino-N-isobutylbenzamide (8b) [24]. Compound wassynthesized according to general procedure, using the followingreagents: 2-methylpropan-1-amine (7b) (400 mL, 4.00 mmol, 2.00eq.), 3-aminobenzoic acid (274 mg, 2.00 mmol, 1.00 eq.), DIPEA(700 mL, 4.00 mmol, 2.00 eq.), EDC*HCl (767 mg, 4.00mmmol, 2.00eq.), HOBt (81 mg, 0.60 mmol, 0.3 eq.) and DMF (7 mL). Yellow oil,274 mg, 100% yield. 1H NMR (400 MHz, CDCl3) d 7.17 (t, J 7.6 Hz,1H), 7.12 (t, J 1.8 Hz, 1H), 7.05 (dd, J 7.6, 1.6, 0.4 Hz, 1H), 6.77(ddd, J 8.0, 2.4, 1.2 Hz, 1H), 6.31 (s br, 1H), 3.83 (s br, 2H), 3.24 (dd,J 6.0, 0.8 Hz, 2H), 1.88 (septet, J 6.8 Hz, 1H), 0.96 (d, J 6.8 Hz,6H) ppm. LC-MS calculated for C11H16N2O [MH]: 193.13, found193.05.
References:
van Veldhoven, Jacobus P.D.;Campostrini, Giulia;van Gessel, Constantijn J.E.;Ward-van Oostwaard, Dorien;Liu, Rongfang;Mummery, Christine L.;Bellin, Milena;IJzerman, Adriaan P. [European Journal of Medicinal Chemistry,2021,vol. 212]