
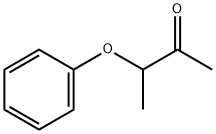
[(3-aminobutan-2-yl)oxy]benzene synthesis
- Product Name:[(3-aminobutan-2-yl)oxy]benzene
- CAS Number:6437-84-9
- Molecular formula:C10H15NO
- Molecular Weight:165.23
6437-85-0
23 suppliers
$90.00/100mg
![[(3-aminobutan-2-yl)oxy]benzene](/CAS/20200515/GIF/6437-84-9.gif)
6437-84-9
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydroxide;hydroxylamine hydrochloride;sodium acetate in ethanol;water;
Steps:
65.75 Examples 65-75
3-Phenoxybutan-2-one (69.6 g.) and ethanol (300 ml.) were added to a solution of hydroxylamine hydrochloride (70 g.) and fused sodium acetate (70 g.) in water (130 ml.). The resulting solution was refluxed for 31/2 hours and evaporated to near dryness under reduced pressure. The residue was partitioned between water and ether. The ether extract was washed with water, dried over anhydrous potassium carbonate and evaporated. The residue was distilled to give a fraction, b.p. 154°-155°/14 mm. which crystallized. Recrystallisation from petroleum ether (b.p. 60°-80°) gave pure 3-phenoxybutan-2-one oxime, m.p. 65°-67°. A solution of 3-phenoxybutan-2-one oxime (28.8 g.) in dry ether (150 ml.) was added dropwise, over 45 minutes, to a stirred, refluxing mixture of lithium aluminium hydride (6.3 g.) in dry ether (100 ml.). Stirring and heating were continued for 4 hours and the mixture was cooled and treated cautiously with water (6.3 ml.) followed by 15% aqueous sodium hydroxide (6.3 ml.), and finally, water (18.9 ml.). The mixture was stirred for 30 minutes and then filtered. The filtrate was extracted with 2N-hydrochloric acid (150 ml.) and the acid extract was made strongly basic with sodium hydroxide and extracted with ether. The ether extract was dried over anhydrous potassium carbonate, evaporated, and distilled to give a fraction, b.p. 118°-124°/14 mm. Redistillation gave pure 2-phenoxybut-3-ylamine, b.p. 116°-118°/14 mm. Also by Method C (ii) was prepared 2-3'-methylphenoxybut-3-ylamine, b.p. 128°-130°/15 mm. The intermediate ketone had b.p. 120°-122°/14 mm. and the derived oxime had b.p. 161°-162°/13 mm.
References:
US3987158,1976,A