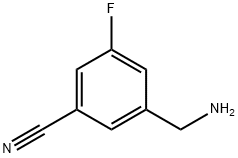
3-Aminomethyl-5-fluoro-benzonitrile synthesis
- Product Name:3-Aminomethyl-5-fluoro-benzonitrile
- CAS Number:1261450-40-1
- Molecular formula:C8H7FN2
- Molecular Weight:150.15
216976-30-6
151 suppliers
$7.00/250mg

1261450-40-1
15 suppliers
inquiry
Yield:1261450-40-1 71%
Reaction Conditions:
with ammonium hydroxide in tetrahydrofuran at 85; for 2 h;
Steps:
32.1
Step 1: 3-(Aminomethyl)-5-fluorobenzonitrileTo 3-(bromomethyl)-5-fluorobenzonitrile (Intermediate 14, step 1 , 10 g, 0.47 mol) in THF (50 mL) was added ammonium hydroxide solution 25% (2.0 L). The resulting mixture was stirred for 2 h at 85°C. It was then cooled down to RT and extracted with dichloromethane. The organic layer was washed with water, brine, dried and concentrated, affording the title compound as a slight yellow liquid (5 g, 71 %). 1H NMR (DMSO-d6, 400 MHz): δ 7.6 (d, J = 6.6 Hz, 1 H), 7.57 (t, J = 0.6 Hz, 1 H), 7.55 (s, 1 H), 3.74 (d, 2H), 1.97 (s, 2H). LC/MS (Method A) 151 .0 (M+H)+. HPLC (Method A) Rt: 0.88 min (Purity 98%).
References:
WO2012/4287,2012,A1 Location in patent:Page/Page column 87
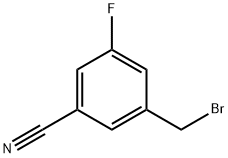
853368-35-1
32 suppliers
inquiry

1261450-40-1
15 suppliers
inquiry