
3-(Benzo[D][1,3]Dioxol-6-Yl)Propane-Hydrazide synthesis
- Product Name:3-(Benzo[D][1,3]Dioxol-6-Yl)Propane-Hydrazide
- CAS Number:88368-72-3
- Molecular formula:C10H12N2O3
- Molecular Weight:208.21
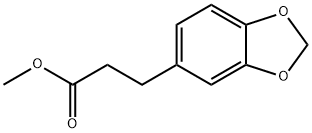
57906-98-6
0 suppliers
inquiry
![3-(Benzo[D][1,3]Dioxol-6-Yl)Propane-Hydrazide](/CAS/GIF/88368-72-3.gif)
88368-72-3
16 suppliers
inquiry
Yield:88368-72-3 77%
Reaction Conditions:
with hydrazine hydrate in dichloromethane at 20; for 0.5 h;Inert atmosphere;
Steps:
General procedure for the synthesis of hydrazides 11, 14,16 and 19
General procedure: The corresponding carboxylic acid (1.0 mmol) andoxalyl chloride (10 mmol) were added to a 10 mL roundbottom flask equipped with a magnetic stirring bar. Arubber septum was used and the reaction was kept under a dry N2 atmosphere. The resulting solution was subjectedto stirring at room temperature for about 0.5 h and theevolution of the reaction was accompanied by TLC(indirectly by the reaction of an aliquot with methanolleading to the spontaneous formation of the correspondingmethyl ester). After the removal of oxalyl chlorideexcess the residue was dissolved in dry dichloromethane(3.0 mL). The resulting solution was added dropwiseto an ice-cooled mixture of hydrazine monohydrate(15 mmol) and dichloromethane (5 mL) placed in a 25 mLround bottom flask equipped with a stirring bar. A rubberseptum was used and the reaction was kept under a dryN2 atmosphere. After reaching room temperature (about30 min), the solvent was removed under reduced pressure.The product precipitated after the addition of ice waterto the previously obtained residue and was then filtered,leading to the formation of the desired hydrazides as asolid material (72-91% yield). All the hydrazides obtained,e.g., 11, 14, 16 and 19, were characterized by LRMS, 1Hand 13C NMR.
References:
Franklim, Tatiany N.;Freire-De-Lima, Leonardo;Chaves, Otávio A.;LaRocque-De-Freitas, Isabel F.;da Silva-Trindade, Joana D.;Netto-Ferreira, José C.;Freire-De-Lima, Célio G.;Decoté-Ricardo, Debora;Previato, José O.;Mendon?a-Previato, Lucia;De Lima, Marco E.F. [Journal of the Brazilian Chemical Society,2019,vol. 30,# 7,p. 1378 - 1394]
![3-BENZO[1,3]DIOXOL-5-YL-PROPIONIC ACID ETHYL ESTER](/CAS/GIF/7116-48-5.gif)
7116-48-5
4 suppliers
inquiry
![3-(Benzo[D][1,3]Dioxol-6-Yl)Propane-Hydrazide](/CAS/GIF/88368-72-3.gif)
88368-72-3
16 suppliers
inquiry
![3-BENZO[1,3]DIOXOL-5-YL-PROPAN-1-OL](/CAS/GIF/7031-03-0.gif)
7031-03-0
12 suppliers
inquiry
![3-(Benzo[D][1,3]Dioxol-6-Yl)Propane-Hydrazide](/CAS/GIF/88368-72-3.gif)
88368-72-3
16 suppliers
inquiry
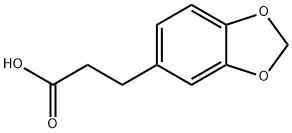
2815-95-4
111 suppliers
$22.00/1g
![3-(Benzo[D][1,3]Dioxol-6-Yl)Propane-Hydrazide](/CAS/GIF/88368-72-3.gif)
88368-72-3
16 suppliers
inquiry

2373-80-0
179 suppliers
$12.00/10g
![3-(Benzo[D][1,3]Dioxol-6-Yl)Propane-Hydrazide](/CAS/GIF/88368-72-3.gif)
88368-72-3
16 suppliers
inquiry