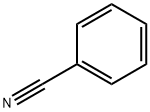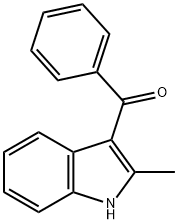
3-BENZOYL-2-METHYLINDOLE synthesis
- Product Name:3-BENZOYL-2-METHYLINDOLE
- CAS Number:26211-73-4
- Molecular formula:C16H13NO
- Molecular Weight:235.28
95-20-5
383 suppliers
$5.00/10g

98-88-4
579 suppliers
$10.00/5g

26211-73-4
2 suppliers
inquiry

78593-46-1
0 suppliers
inquiry
Yield:26211-73-4 95% ,78593-46-1 5%
Reaction Conditions:
with MIL-53(Al)(at)SiO2(at)Fe3O4 in dodecane;dichloromethane at 25; for 8 h;Catalytic behavior;Friedel-Crafts Acylation;Reagent/catalyst;Temperature;Solvent;Time;
Steps:
2.3. Catalytic activity evaluations
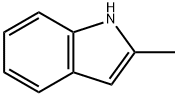
Friedel-Crafts acylation reaction of 2-methylindole with ben-zoyl chloride was carried out in a flask reactor with a condenser. Acertain amount of 2-methylindole, benzoyl chloride, n-dodecane,dichloromethane and the catalysts (the content of the catalyst wasused in reference to the molar ratio of 2-methylindole, n1:ncatalyst)were added in the flask with magnetic stirring. After reaction, thecatalysts were separated from the solvent by an external magnet.The above layer of liquid was detected through GC (Beijing Beifen-Ruili Analytical Instrument (Group) Co. Ltd, SP-2100, HJ.PONA,50 m × 0.20 mm × 0.50 m). The conversion of 2-methylindole (X),the selectivity (S3) and the yield (Y3) of 3-acetylindole, the selec-tivity (S4) and the yield (Y4) of N-acetylindole were all calculatedwith n-dodecane as an internal standard. After pouring the liquidfrom the flask, the solid catalyst was washed with dichloromethaneand separated by an external magnet. Then the fresh reagents wereadded to the flask reactor and used for the next run. In order tostudy the effect of different indole derivatives on the Friedel-Craftsacylation reaction, different substrates (indole with different sub-stituent groups, such as, H, CH3, 5-OCH3, 6-NO2) were usedto perform the reaction. Besides, cyclohexane, CHCl3, nitroben-zene were used as alternative solvents in Friedel-Crafts acylationreaction.
References:
Jiang, Sai;Yan, Junlei;Habimana, Fabien;Ji, Shengfu [Catalysis Today,2016,vol. 264,p. 83 - 90]

95-20-5
383 suppliers
$5.00/10g
201230-82-2
1 suppliers
inquiry
369-57-3
42 suppliers
inquiry

26211-73-4
2 suppliers
inquiry