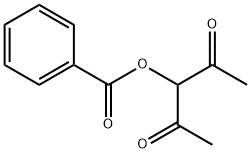
3-(Benzoyloxy)-2,4-pentanedione synthesis
- Product Name:3-(Benzoyloxy)-2,4-pentanedione
- CAS Number:4620-47-7
- Molecular formula:C12H12O4
- Molecular Weight:220.22
Yield:4620-47-7 99.82%
Reaction Conditions:
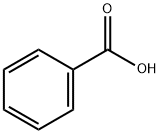
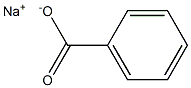
Stage #1: benzoic acidwith potassium hydroxide in N,N-dimethyl-formamide at 50; for 1 h;
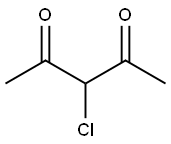
Stage #2: 3-chloropentane-2,4-dione in N,N-dimethyl-formamide at 50;
Steps:
1.6 Step 6: Preparation of (1-acetyl-2-oxo-propyl) benzoate
[0213] A solution of benzoic acid (75 g, 614.14 mmol, 93.75 mL, 1 eq) and KOH (34.46 g, 614.14 mmol, 1 eq) in DMF (700 mL) was stirred at 50°C for 1 h. Then 3-chloropentane-2,4-dione (82.64 g, 614.14 mmol, 73.13 mL, 1 eq) was added into the above solution at 50°C and stirred at 50°C for 13 hr. TLC indicated the chloride was consumed and one major new spot with lower polarity was detected. The reaction mixture was partitioned between MTBE 500 mL and water 500 mL. The organic phase was separated, washed with brine 500 mL, dried overNa2SO4, filtered. The filtrate was concentrated under reduced pressure to give (1-acetyl-2-oxo-propyl) benzoate (135 g, 613.02 mmol, 99.82% yield) as yellow oil.[0214]1H NMR (400 MHz, DMSO-d6) δ = 8.09 (d, J = 7.6 Hz, 2H), 7.78 - 7.71 (m, 1H), 7.63 - 7.57 (m, 2H), 5.93 (s, 1H), 2.34 (s, 6H).
References:
WO2021/236893,2021,A1 Location in patent:Paragraph 0213-0214