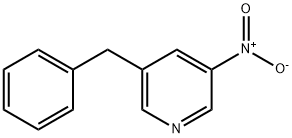
3-Benzyl-5-nitropyridine synthesis
- Product Name:3-Benzyl-5-nitropyridine
- CAS Number:1428065-77-3
- Molecular formula:C12H10N2O2
- Molecular Weight:214.22
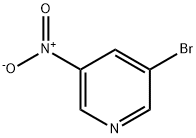
15862-30-3
141 suppliers
$11.00/250mg
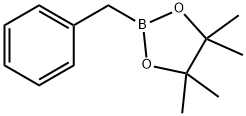
87100-28-5
153 suppliers
$25.00/500mg

1428065-77-3
5 suppliers
inquiry
Yield:1428065-77-3 37%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium phosphate in water at 90; for 2 h;Microwave irradiation;Suzuki Coupling;
Steps:
17.1 Step 1
To a solution of 3-bromo-5-nitropyridine (LXXVII) (295 mg, 1.45 mmol) in dioxane (14 mL) was added 2-benzyl-4,4,5,5-tetramethyl-l,3,2-dioxaborolane (LXXVIII) (420 1.89 mmol), PdCl2(dppf)2, (120 mg, 0.15 mmol) and 2M aqueous K3PO4 (2.2 mL, 4.36 mmol). The reaction was microwaved at 90°C for 2h. The reaction was cooled and the organic phase was separated, dried over MgS04 and evaporated under vacuum. The residue was purified by silica gel column chromatography (100% hexane→ 6:94 EtOAc:hexane) to give 3-benzyl-5-nitropyridine (LXXIX) as brown oil (117 mg, 0.54 mmol, 37% yield). 1H NMR (DMSO-d6) δ ppm 4.16 (s, 2H), 7.21-7.25 (m, 1H), 7.31-7.33 (m, 4H), 8.45-8.46 (m, 1H), 8.93 (d, J=2Hz, 1H), 9.21 (d, J=3Hz, 1H); ESIMS found for C12Hi0N2O2 mlz 215 (M+H).
References:
WO2013/40215,2013,A1 Location in patent:Paragraph 00300
104-53-0
267 suppliers
$6.00/5g
14150-94-8
147 suppliers
$17.67/250mgs:

1428065-77-3
5 suppliers
inquiry