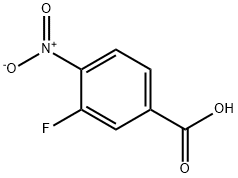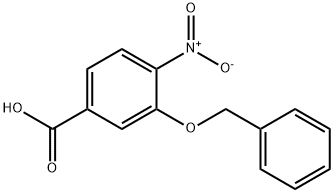
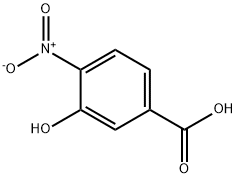
3-(Benzyloxy)-4-nitrobenzenecarboxylic acid synthesis
- Product Name:3-(Benzyloxy)-4-nitrobenzenecarboxylic acid
- CAS Number:14617-29-9
- Molecular formula:C14H11NO5
- Molecular Weight:273.24
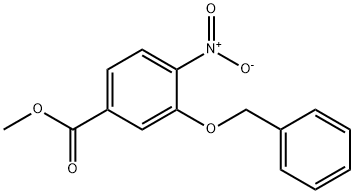
209528-69-8
15 suppliers
inquiry

14617-29-9
12 suppliers
inquiry
Yield:14617-29-9 96%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;methanol at 60; for 3 h;
Steps:
1b 3-(benzyloxy)-4-nitrobenzoic acid (3)
Example 1b
3-(benzyloxy)-4-nitrobenzoic acid (3)
Cmpd 2 (1.53 g, 1.0 eq) was dissolved in THF (52.8 mL) and MeOH (26.4 mL) in a round bottom flask, followed by addition of 2N NaOH (13.2 mL, 5.0 eq).
The reaction was heated at 60° C. for 3 hours, occasionally opening the flask to release pressure.
The reaction was monitored by TLC (5% MeOH:95% DCM).
The resulting mixture was concentrated, and the residue was dissolved in water and acidified to pH=2-3 with 1N HCl.
The acidic solution was extracted with AcOEt 3*, dried over Na2SO4, filtered and concentrated to afford Cmpd 3 as a light yellowish solid (1.25 g, 96% yield). Analytic: 1H NMR (DMSO d6, 500 mHz): δ 7.99 (d, 1H), 7.87 (s, 1H), 7.66 (dd, 1H), 7.46-7.40 (m, 4H), 7.33 (m, 1H), 5.39 (s, 2H). LCMS (C18, 2-98% CH3CN in 0.1% TFA/H2O over 6 min); Calculated mass for C14H11NO5 (M+H)+274.06. found by LC-MS 274.
References:
US9217022,2015,B2 Location in patent:Page/Page column 42
713-52-0
168 suppliers
$10.00/1g

14617-29-9
12 suppliers
inquiry
619-14-7
290 suppliers
$10.00/5g

14617-29-9
12 suppliers
inquiry

100-39-0
426 suppliers
$10.00/10g

14617-29-9
12 suppliers
inquiry