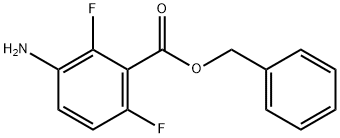
{3-[(Benzyloxy)carbonyl]-2,4-difluorophenyl}amine synthesis
- Product Name:{3-[(Benzyloxy)carbonyl]-2,4-difluorophenyl}amine
- CAS Number:918523-44-1
- Molecular formula:C14H11F2NO2
- Molecular Weight:263.24
Yield:918523-44-1 79.7%
Reaction Conditions:
Stage #1: 2,4-difluorophenylaminewith n-butyllithium in tetrahydrofuran;hexane; for 0.5 h;Cooling with acetone-dry ice;
Stage #2: with 1,2-bis-(chlorodimethylsilyl)ethane in tetrahydrofuran;hexane; for 1 h;Cooling with acetone-dry ice;
Stage #3: benzyl chloroformatewith hydrogenchloride;n-butyllithium;potassium carbonatemore than 3 stages;
Steps:
1.1
To 2,4-difluoro-phenylamine (42, 5.11 mL, 50.7 mmol) in tetrahydrofuran (250 mL), cooled with dry ice/acetone bath under an atmosphere of nitrogen, was added n-butyllithium (1.60 M in hexane, 34.0 mL, 54.4 mmol) slowly. After 30 minutes, l,2-Bis-(chloro-dimethyl-silanyl)-ethane (11.5 g, 53.4 mmol) dissolved in tetrahydrofuran (40.0 mL) was added to the reaction slowly. After 1 hour, n-butyllithium (1.60 M in hexane, 31.9 mL, 51.0 mmol) was added slowly to the reaction. The reaction was stirred at -78 0C for 30 minutes and then allowed to warm to room temperature over 40 minutes. The reaction was cooled to -78 0C, followed by addition of n-butyllithium (1.60 M in hexane, 35.1 mL, 56.2 mmol) slowly. After 70 minutes, benzyl chloroformate (7.97 mL, 55.8 mmol) was added to the reaction. The reaction mixture was stirred at -78 °C overnight followed by addition of 2 N HCl (120 mL). The reaction was allowed to warm to room temperature for 2 hours. The EPO
References:
WO2007/2325,2007,A1 Location in patent:Page/Page column 74-75
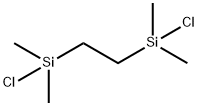
13528-93-3
189 suppliers
$6.00/1g
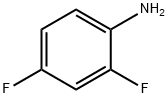
367-25-9
489 suppliers
$5.00/5g

501-53-1
392 suppliers
$10.00/1g
![{3-[(Benzyloxy)carbonyl]-2,4-difluorophenyl}amine](/CAS/GIF/918523-44-1.gif)
918523-44-1
11 suppliers
inquiry