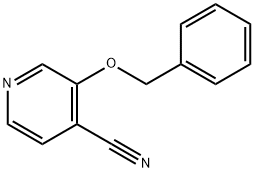
3-(Benzyloxy)pyridine-4-carbonitrile synthesis
- Product Name:3-(Benzyloxy)pyridine-4-carbonitrile
- CAS Number:78790-76-8
- Molecular formula:C13H10N2O
- Molecular Weight:210.23
68325-15-5
246 suppliers
$6.00/100mg

100-51-6
1429 suppliers
$5.00/100g

78790-76-8
19 suppliers
inquiry
Yield:78790-76-8 84.03%
Reaction Conditions:
Stage #1: benzylic alcoholwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 0.583333 h;Inert atmosphere;
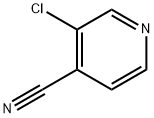
Stage #2: 3-chloropyridine-4-carbonitrile in N,N-dimethyl-formamide;mineral oil at 20;Inert atmosphere;
Steps:
1.2; 25.3 25.3. Synthesis of 3-(benzyloxy)pyridine-4-carbonitrile
A solution of benzyl alcohol (9.37 g, 86.611 mmol, 1.20 equiv) in DMF (70.00 mL) was treated with NaH (3.75 g, 93.829 mmol, 1.30 equiv, 60%) for 5 min at 0 degrees C under nitrogen atmosphere at room temperature. The resulting mixture was stirred for 30 min at room temperature under nitrogen atmosphere. To the above mixture was added 3-chloropyridine-4-carbonitrile (10.00 g, 72.176 mmol, 1.00 equiv) in portions over 5 min at room temperature. The resulting mixture was stirred for additional overnight at room temperature. The reaction was quenched by the addition of saturated NH4Cl aqueous solution (10 mL) at room temperature. The resulting mixture was extracted with EtOAc (3 x 200 mL). The combined organic layers were washed with brine (2 x 200 mL), dried over anhydrous Na2SO4. After filtration, the filtrate was concentrated under reduced pressure. This resulted in 3-(benzyloxy)pyridine-4- carbonitrile (15 g, 84.03%) as a yellow solid. LC-MS: M+H found: 211.1.
References:
WO2022/66734,2022,A1 Location in patent:Page/Page column 302-303; 334-335

14906-59-3
240 suppliers
$27.00/5g

78790-76-8
19 suppliers
inquiry