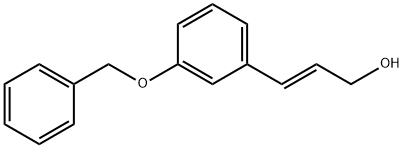
3-BenzyloxycinnaMyl alcohol synthesis
- Product Name:3-BenzyloxycinnaMyl alcohol
- CAS Number:155697-42-0
- Molecular formula:C16H16O2
- Molecular Weight:240.3

208836-69-5
1 suppliers
inquiry

155697-42-0
34 suppliers
$13.00/1g
Yield:155697-42-0 98.2%
Reaction Conditions:
Stage #1: 3-(3'-benzyloxyphenyl)-(E)-propenoic acid ethyl esterwith diisobutylaluminium hydride in toluene at -10 - 0;Inert atmosphere;
Stage #2: with ammonium chloride in toluene at 5;Product distribution / selectivity;
Steps:
5
Example 5Preparation of (2£)-3-(3-benzyloxyphenyl)prop-2-en-l-ol (general formula IV: R = CH2Ph); A solution of 103.1 g (0.365 mol) of ethyl-3 '-benzyloxy-E cinnamate in dry toluene (730 ml) is cooled in an ice bath (ice + salt) under argon atmosphere to - 10°C, and then, a 1 M solution of diisobutylalane in toluene is added dropwise (temperature range -5 to 0°C) within 50 min. The yellowish solution is stirred at about -3°C for 2.5 h; the course of the reaction is checked using TLC, the cooling is switched off, and slow stirring continues overnight.The reaction mixture cooled in an ice bath to 5°C is added gradually under stirring to 500 ml of a cooled saturated solution of ammonium chloride. Then, 1 N hydrochloric acid (500 ml) and, thereafter, concentrated hydrochloric acid (145 ml) are added. Methyl tert-butyl ether (MTBE; 400 ml) is added and the mixture is stirred for another 30 minutes. The aqueous phase is separated and shaken with MTBE (400 ml, 150 ml). The combined organic portions are washed with 0.5 N sodium hydroxide (380 ml), water (380 ml), and brine (350 ml). After filtration, the solution is evaporated in a rotating vacuum evaporator, during which a product crystallizes. 86.17 g (98.2 %) of the alcohol of general formula II: R = CH2Ph) is obtained, sufficiently pure for using in the following step, melting point = 60.2 - 63.7°C.-NMR (CDCI3) δ 1.40 (1 H, bs), 4.32 (2H, d, J = 5.6 Hz), 5.08 (2H, s), 6.35 (1 H, dt, J = 5.6, 15.9 Hz), 6.59 (1 H, d, J = 15.9 Hz), 6.88 (1H, dd, J = 2.5, 8.3 Hz), 6.98-7.01 (2H, m), 7.20-7.39 (6H, m).
References:
WO2012/89181,2012,A1 Location in patent:Page/Page column 24

100-39-0
433 suppliers
$10.00/10g

155697-42-0
34 suppliers
$13.00/1g

96251-92-2
18 suppliers
inquiry

155697-42-0
34 suppliers
$13.00/1g
1700-37-4
248 suppliers
$9.00/1g

155697-42-0
34 suppliers
$13.00/1g
![2-Propenoic acid, 3-[3-(phenylmethoxy)phenyl]-, phenylmethyl ester, (2E)-](/CAS/20210305/GIF/1026884-98-9.gif)
1026884-98-9
0 suppliers
inquiry

155697-42-0
34 suppliers
$13.00/1g