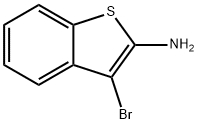
3-Bromo-1-benzothiophen-2-amine synthesis
- Product Name:3-Bromo-1-benzothiophen-2-amine
- CAS Number:344747-99-5
- Molecular formula:C8H6BrNS
- Molecular Weight:228.11
![3-BROMO-2-NITRO-BENZO[B]THIOPHENE](/CAS/GIF/17402-78-7.gif)
17402-78-7
41 suppliers
inquiry

344747-99-5
3 suppliers
inquiry
Yield: 99%
Reaction Conditions:
with iron;acetic acid in ethanol at 60; for 1 h;
Steps:
15 Preparation of Compound 8-2
Compound 8-1 9g (34.87mmol) was stirred with 300mL of ethanol, put into an iron powder (Fe Powder) 6.03g (108.1mmol) for 10 minutes at room temperature respectively. 30mL acetic acid was added dropwise thereto slowly and the mixture was refluxed for 1 hour at 60°C. After completion of the reaction after cooling to room temperature, H2O After filtration, the solid that was generated by the addition of H2O and washed with hexane to give 7.9g of the desired compound 8-2 (99%).
References:
Heesung Material Co., Ltd;Lee, Jong Hyon;Noh, Young-seok;Park, Gon Yu;Kim, Dong-jun;Kim, Gi Young;Choe, Jin Seok;Choe, Dai Hyok;Um, Song Jean;Lee, Joo Dong KR2016/1702, 2016, A Location in patent:Paragraph 0267-0268; 0271-0272
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

344747-99-5
3 suppliers
inquiry
![BENZO[B]THIOPHENE-2-CARBONYL CHLORIDE](/CAS/GIF/39827-11-7.gif)
39827-11-7
115 suppliers
$17.00/1g

344747-99-5
3 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

344747-99-5
3 suppliers
inquiry
![tert-butyl benzo[b]thiophen-2-ylcarbaMate](/CAS/GIF/89673-36-9.gif)
89673-36-9
48 suppliers
$11.00/100mg

344747-99-5
3 suppliers
inquiry