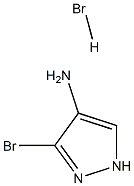
3-Bromo-1H-pyrazol-4-amine hydrobromide synthesis
- Product Name:3-Bromo-1H-pyrazol-4-amine hydrobromide
- CAS Number:1374320-70-3
- Molecular formula:C3H5Br2N3
- Molecular Weight:242.8999
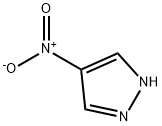
2075-46-9
312 suppliers
$6.00/5g

1374320-70-3
4 suppliers
inquiry
Yield:1374320-70-3 84%
Reaction Conditions:
with hydrogen bromide;hydrogen;Pd/Al2O3 in ethanol;water; under 517.162 Torr; for 36 h;
Steps:
16
Example 16 Preparation of 5-bromo-1H-pyrazol-4-amine, HBr A mixture of 4-nitro-1H-pyrazole (10 g, 88 mmol) and 5% palladium on Al2O3 (1 g) in a mixture of ethanol (150 mL) and 50% aqueous HBr (50 mL) was shaken in a Par apparatus under hydrogen (10 psi) for 36 h. The mixture was filtered and the catalyst washed with ethanol. The filtrate was concentrated in vacuo to give a white solid. This solid was suspended in 10 mL of ethanol. After swirling the flask for 5 min, ether was added to complete the crystallization. The solid was filtered, was washed with ether and dried under high vacuum to afford 5-bromo-1H-pyrazol-4-amine, HBr (18.1 g, 84% yield) as a white solid: mp 248° C. dec; 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.00 (s, 1H), 7.79 (s, 1H).
References:
US2012/110702,2012,A1 Location in patent:Page/Page column 21