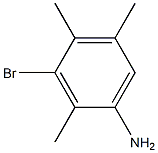
3-Bromo-2,4,5-trimethylaniline synthesis
- Product Name:3-Bromo-2,4,5-trimethylaniline
- CAS Number:18087-53-1
- Molecular formula:C9H12BrN
- Molecular Weight:214.1023
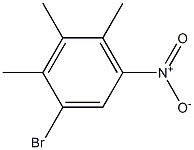
18087-51-9
1 suppliers
inquiry

18087-53-1
4 suppliers
inquiry
Yield:18087-53-1 66%
Reaction Conditions:
with triethylamine hydrochloride;zinc in N,N-dimethyl-formamide at 105;
Steps:
2 Step 2
A suspension of 3-bromo-1,2,4-trimethyl-5-nitrobenzene (63) (3.0 g, 12.5 mmol), zinc dust (3.7 g, 56.1 mmol) and triethylamine HCl (9.4 g, 68.5 mmol) in DMF (42 mL) was heated at 105° C. overnight.
The reaction mixture was allowed to cool to room temperature, and filtered through Celite.
The filtrate was diluted with EtOAc and the organic layer was washed with brine (twice), dried over magnesium sulfate, filtered, and concentrated under reduced pressure.
The crude product was purified by silica gel chromatography (ISCO 24 g silica) and eluted with EtOAc/heptane (0-40%) and gave 3-bromo-2,4,5-trimethylaniline (64) as brown oil which gave a brown solid upon standing (1.8 g, 66% yield). LCMS (ESI) m/z 214, 216 (M+H).
References:
US2019/233440,2019,A1 Location in patent:Paragraph 0268; 0269
610-91-3
80 suppliers
$25.00/1g

18087-53-1
4 suppliers
inquiry
95-72-7
153 suppliers
$42.00/100g

18087-53-1
4 suppliers
inquiry
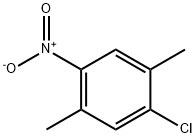
34633-69-7
71 suppliers
$11.00/1g

18087-53-1
4 suppliers
inquiry
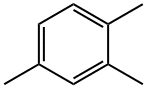
95-63-6
265 suppliers
$15.69/250mg

18087-53-1
4 suppliers
inquiry