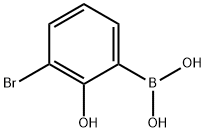
3-Bromo-2-hydroxyphenyl boronic acid synthesis
- Product Name:3-Bromo-2-hydroxyphenyl boronic acid
- CAS Number:89488-24-4
- Molecular formula:C6H6BBrO3
- Molecular Weight:216.82
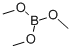
121-43-7
344 suppliers
$15.00/25ml

608-33-3
279 suppliers
$10.00/5g

89488-24-4
31 suppliers
$436.00/10g
Yield:89488-24-4 47%
Reaction Conditions:
Stage #1: 2,6-dibromophenolwith n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Stage #2: Trimethyl borate in tetrahydrofuran;hexane; for 2 h;
Steps:
1
Compound a1-2,6 dibromophenol (9.47 g, 37.6 mmol)Dissolved in tetrahydrofuran (140 mL),Add hexane solvent and 2.5M at -78 °CAfter n-butyl lithium (18 mL, 45.1 mmol),Stir for 1 hour.Then slowly add trimethyl borate (13 mL, 56.4 mmol)After that, stir for 2 hours.Then add 2M hydrochloric acid to neutralize,The product was extracted with ethyl acetate and water.Recrystallization from dichloromethane and hexane gave compound b1 (4.10 g, 47%).Weighed compound b1 (5.82 g, 25.1 mmol),Compound c1 (7.13 g, 25.1 mmol),Tetrakistriphenylphosphine palladium (11g, 10mmol)And potassium carbonate (8.42 g, 60.9 mmol),The weighed reaction was dissolved in toluene (1 L) / EtOH (200 mL) /In the solvent of distilled water (200 mL),Heat at 90 ° C for 2 hours.Weighed compound d1 (12.50 g, 38 mmol),Add KOH and Cu powder,And heating at 200 ° C to carry out the reaction,Compound e1 was obtained.
References:
CN108676034,2018,A Location in patent:Paragraph 0074; 0075; 0076; 0077
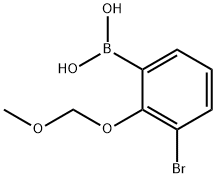
1431330-19-6
4 suppliers
inquiry

89488-24-4
31 suppliers
$436.00/10g

608-33-3
279 suppliers
$10.00/5g

89488-24-4
31 suppliers
$436.00/10g
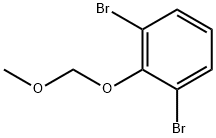
142273-81-2
7 suppliers
inquiry

89488-24-4
31 suppliers
$436.00/10g