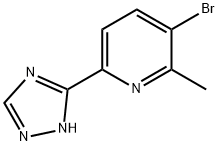
3-bromo-2-methyl-6-(1H-1,2,4-triazol-3-yl)pyridine synthesis
- Product Name:3-bromo-2-methyl-6-(1H-1,2,4-triazol-3-yl)pyridine
- CAS Number:1228014-23-0
- Molecular formula:C8H7BrN4
- Molecular Weight:239.07
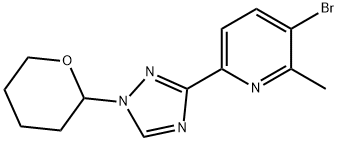
1228014-21-8
4 suppliers
inquiry

1228014-23-0
8 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in methanol;water at 20; for 16 h;
Steps:
S-33.1 Step-1: Preparation of 3-bromo-2-methyl-6-(1H-1,2,4-triazol-3-yl)pyridine
To 3-bromo-2-methyl-6-(1-(tetrahydro-2H-pyran-2-yl)-1H-1,2,4-triazol-3-yl)pyridine (1.6 g, 4.95 mmol) was added 2M-HCl in MeOH (15 mL) and resulting mixture was stirred at RT for 16 h. The reaction was monitored by TLC & LC-MS. After completion, the mixture was concentrated under reduced pressure to afford the title compound. LC-MS 239 [M+H]+Step-2a: Preparation of 2-(6-iodohexyl)isoindoline-1,3-dioneTo a solution of potassium 1,3-dioxoisoindolin-2-ide (5.0 g, 26.99 mmol) in DMF (50 mL) was added 1,6-Diiodohexane (22.8 g, 67.49 mmol) and the mixture was heated at 70 °C for 2.5 h. The reaction was monitored by TLC. After completion, the mixture was diluted with H2O (100 mL) and extracted with EtOAc (200 mL × 2). The combined organic layers were washed with brine (100 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to obtain a crude which was purified by CombiFlash chromatography to afford the title compound. LC-MS 358 [M+H]+
References:
WO2019/222272,2019,A1 Location in patent:Page/Page column 254; 255
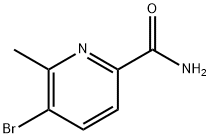
1228014-22-9
17 suppliers
inquiry
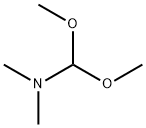
4637-24-5
598 suppliers
$5.00/25g

1228014-23-0
8 suppliers
inquiry
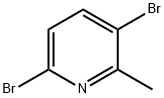
39919-65-8
300 suppliers
$5.00/1g

1228014-23-0
8 suppliers
inquiry
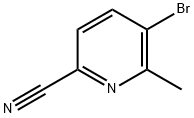
1173897-86-3
105 suppliers
inquiry

1228014-23-0
8 suppliers
inquiry