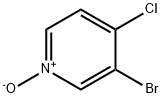
3-BroMo-4-chloro-pyridine 1-oxide synthesis
- Product Name:3-BroMo-4-chloro-pyridine 1-oxide
- CAS Number:99839-30-2
- Molecular formula:C5H3BrClNO
- Molecular Weight:208.44

89364-04-5
134 suppliers
$45.00/1g

99839-30-2
17 suppliers
inquiry
Yield:99839-30-2 97%
Reaction Conditions:
Stage #1: 3-bromo-4-nitropyridinewith hydrogenchloride in methanol at 0 - 20; for 7 - 10 h;
Stage #2: with sodium hydroxide in water;
Steps:
3
To a well stirred solution of 3-bromo-4-nitro pyridine (12.Og, 54.794 mmol) in methanol (120 mL) was bubbled dry hydrochloride gas at about 0-50C for about 1-2-hours. The reaction mixture was then stirred at ambient temperature for about 6-8 hours. The solvent was evaporated under reduced pressure, the residue obtained was diluted with 20% aqueous solution of sodium hydroxide (100 mL) and extracted with dichloromethane (3 x 100 mL). The combined organic layer was washed with water and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give 3-bromo-4-chloro-pyridine-N- oxide as an off-white crystalline solid. Yield: >97%, m.p.: 147-1490C.IR (KBr):- 3436, 3078, 3054, 1449, 1414, 1274, 1243, 1150, 1042, 900, 670 cm'1. 1H NMR (300 MHz, DMSOd6): d 7.71 (d, IH, J= 6.6 Hz), 8.24 (d, IH, J= 5.1 Hz), 8.75 (s, IH).
References:
WO2008/142542,2008,A2 Location in patent:Page/Page column 20
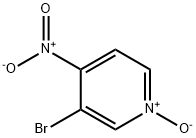
1678-49-5
135 suppliers
$31.00/1g

99839-30-2
17 suppliers
inquiry
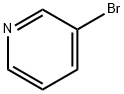
626-55-1
539 suppliers
$5.00/5/ G

99839-30-2
17 suppliers
inquiry
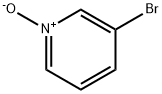
2402-97-3
121 suppliers
$30.00/1g

99839-30-2
17 suppliers
inquiry