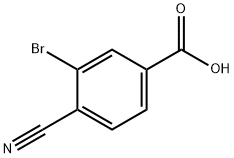
3-BROMO-4-CYANOBENZOIC ACID synthesis
- Product Name:3-BROMO-4-CYANOBENZOIC ACID
- CAS Number:58123-69-6
- Molecular formula:C8H4BrNO2
- Molecular Weight:226.03
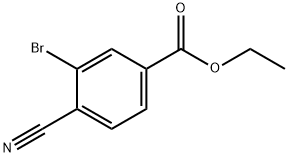
362527-61-5
63 suppliers
inquiry

58123-69-6
47 suppliers
inquiry
Yield:58123-69-6 90%
Reaction Conditions:
Stage #1: ethyl 3-bromo-4-cyanobenzoatewith sodium hydroxide;ethanol;water in tetrahydrofuran; for 20 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;ethanol;water;
Steps:
2
EXAMPLE 2; Preparation of 3-Bromo-4-cyanobenzoic acid; A solution of ethyl 4-amino-3-bromobenzoate (23.5 g, 96.3 mmol) in methylene chloride at -10° C. is treated dropwise with tert-butyl nitrite (14.0 mL, 118.4 mmol), followed by boron trifluoride diethyl etherate (18.4 mL, 146.5 mmol). The reaction mixture is allowed to come to room temperature, stirred for 4 h, diluted with ethyl ether and filtered. The filtercake is dried, dispersed in toluene, cooled to 0° C., treated with a solution of copper (I) cyanide (11.5 g, 129.2 mmol) and sodium cyanide (15.8 g, 323.1 mmol) in water, stirred at 0° C. for 30 min., warmed to 60° C., stirred for 1 h, cooled to room temperature and diluted with EtOAc and water. The organic phase is separated, dried over MgSO4 and concentrated in vacuo. The resultant residue is crystallized from ethyl ether/hexanes to give the ethyl ester of the title compound as an off-white solid, 18.6 g (76% yield). A solution of this ester (8.5 g, 33.6 mmol) in THF is treated with a solution of NaOH (30 mL, 2.5 N) and ethanol, stirred for 20 h, acidified with 2N HCl and extracted with ethyl ether. The ether extracts are combined, dried over MgSO4 and concentrated in vacuo. The resultant residue is crystallized from ethyl ether/hexanes to afford the title compound as a beige solid, 6.81 g (90% yield), identified by HNMR and mass spectral analyses. MS m/e 223 (M-H)+; 1H NMR (400 MHz, DMSO-d6) δ 8.05 (dd, J=9.76, 1.37 Hz 1 H), 8.09 (d, J=8.08 Hz, 1H), 8.29 (d, J=1.37 Hz, 1H).
References:
US2005/282825,2005,A1 Location in patent:Page/Page column 8

942598-44-9
33 suppliers
inquiry

58123-69-6
47 suppliers
inquiry

42872-73-1
150 suppliers
$15.00/5g

58123-69-6
47 suppliers
inquiry
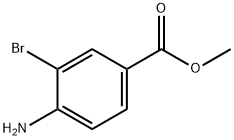
106896-49-5
204 suppliers
$6.00/1g

58123-69-6
47 suppliers
inquiry