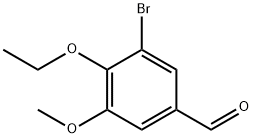
3-BROMO-4-ETHOXY-5-METHOXY-BENZALDEHYDE synthesis
- Product Name:3-BROMO-4-ETHOXY-5-METHOXY-BENZALDEHYDE
- CAS Number:90109-65-2
- Molecular formula:C10H11BrO3
- Molecular Weight:259.1

74-96-4
433 suppliers
$9.00/5g
2973-76-4
304 suppliers
$10.00/1g

90109-65-2
21 suppliers
$45.00/50mg
Yield:90109-65-2 97%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 100; for 2 h;
Steps:
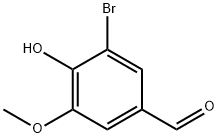
Preparation of 4-(3-bromo-4-ethoxy-5-methoxybenzyl)-7-ethoxyisoquinolin-8-ol hydrochloride 773-Bromo-4-ethoxy-5-methoxybenzaldehvde ANP 36050In an ace pressure tube, 3-bromo-4-hydroxy-5-methoxybenzaldehyde (1 .0 g, 4.33 mmol) was dissolved in anhydrous DMF (10 ml_) and cesium carbonate (1 .48 g, 4.54 mmol) was added at RT. Bromoethane (355 μΙ_, 4.76 mmol) was added and the solution was stirred at 100°C for 2 h. After cooling to RT, the reaction was poured into water (250 ml_) and extracted with Et2O (200 ml_). The organic layer was washed with water (2x50 ml_), brine (50 ml_), dried over MgSO4, and filtered. After evaporation and drying, 3-bromo-4-ethoxy-5-methoxybenzaldehyde ANP 36050 (1 .09 g, 97% yield) was obtained as a white solid.ANP 36050MW: 259.10; Yield: 97%; White solid; Mp (°C): 55.9Rf. 0.8 (CH2CI2:EtOAc = 8:2).1H-NMR (CD3OD, δ): 1 .44 (t, 3H, CH3, J = 7.04 Hz), 3.92 (s, 3H, OMe), 4.19 (q, 2H, CH2, J = 7.06 Hz), 7.38 (s, 1 H, ArH), 7.65 (s, 1 H, ArH), 9.84 (s, 1 H, ArH).13C-NMR (CD3OD, δ): 15.7, 56.2, 69.6, 1 10.0, 1 18.4, 128.8, 132.9, 151 .1 , 154.3, 189.9.MS-ESI m/z (% rel. Int.): 259.0/261 .0 ([MH]+, 100/100).HPLC: Method A, XBridge column, detection UV 254 nm, RT = 5.81 min, peak area 99.9%.
References:
WO2011/151423,2011,A1 Location in patent:Page/Page column 191

2973-76-4
304 suppliers
$10.00/1g

75-03-6
355 suppliers
$11.00/5g

90109-65-2
21 suppliers
$45.00/50mg

121-33-5
1082 suppliers
$9.00/1g

90109-65-2
21 suppliers
$45.00/50mg