
3-Bromo-4-methyl-5-nitropyridine synthesis
- Product Name:3-Bromo-4-methyl-5-nitropyridine
- CAS Number:69872-15-7
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
Yield:69872-15-7 70%
Reaction Conditions:
Stage #1:diethyl malonate with sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2:3-bromo-4-chloro-5-nitropyridine in DMF (N,N-dimethyl-formamide) at 20; for 1 h;
Steps:
94 3-bromo-4-methyl-5-nitropyridine (94a)
3-bromo-4-methyl-5-nitropyridine (94a) Diethyl malonate (3.84 mL, 25.3 mmol) was slowly added to a suspension of sodium hydride (1.01 g of a 60% suspension in oil, 25.3 mmol) in DMF (15 mL) at 0° C. and stirred 30 min until gas evolution ceased. 3-bromo-4-chloro-5-nitropyridine (3.00 g, 12.6 mmol) was added slowly, and the dark reddish-brown solution was stirred at room temperature for 1 hour. The reaction was carefully quenched with water and acidified to pH 1 with 1N HCl. The aqueous mixture was extracted with EtOAc (2*150 mL). The combined organics were washed with water (100 mL) and brine, dried (Na2SO4), filtered, and concentrated in vacuo. The residue was diluted with 4N HCl (50 mL) and the solution refluxed 16 hours. The mixture was cooled in an ice bath and basified to pH 7 with 50% NaOH. The aqueous mixture was extracted with EtOAc (3*100 mL) and the combined organics were washed with brine, dried (MgSO4), filtered and concentrated in vacuo to afford 94a (1.90 g, 70%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ 8.94 (s,1H), 8.97 (s,1H), 2.65 (s, 3H).
References:
PFIZER INC US2005/90529, 2005, A1 Location in patent:Page/Page column 82

69872-14-6
7 suppliers
inquiry

69872-15-7
118 suppliers
inquiry
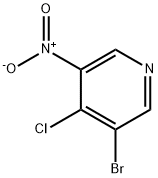
31872-63-6
180 suppliers
inquiry

69872-15-7
118 suppliers
inquiry
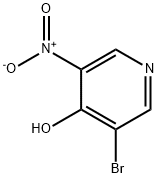
31872-65-8
133 suppliers
$12.00/250mg

69872-15-7
118 suppliers
inquiry
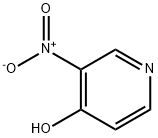
5435-54-1
350 suppliers
$19.00/25g

69872-15-7
118 suppliers
inquiry
