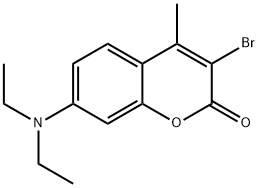
3-bromo-4-methyl-7-(diethylamino)-coumarin synthesis
- Product Name:3-bromo-4-methyl-7-(diethylamino)-coumarin
- CAS Number:92295-93-7
- Molecular formula:C14H16BrNO2
- Molecular Weight:310.19

91-44-1
262 suppliers
$14.00/5g

92295-93-7
7 suppliers
inquiry
Yield:92295-93-7 98%
Reaction Conditions:
Stage #1: 7-diethylamino-4-methylcoumarinwith bromine in chloroform at 20;
Stage #2: with triethylamine in chloroform; for 0.5 h;
Steps:
2.2.1. Synthesis of 3-bromo-7-diethylamino-4-methylcoumarin (2)
To a solution of 7-diethylamino-4-methylcoumarin (100 mg,0.432 mmol) in chloroform (2 mL) was added bromine (0.022 mL,0.432 mmol) in an ice bath. After stirring overnight at room temperature,NEt3 (0.200 mL) was added cautiously. After stirring for further30 min, the reaction mixture was evaporated to dryness and the residuewas purified by flash column chromatography on silica gel (230-400mesh; CHCl3/CH3OH (99:1)) to yield 3-bromo-7-diethylamino-4-methylcoumarin[37] (131 mg, 0.424 mmol, 98%). 1H NMR (400 MHz,CDCl3, , ppm): 1.19 (6H, t, J 7.1, H-11, H-13), 2.50 (3H, s, H-9), 3.40(4H, q, J 7.1, H-10, H-12), 6.46 (1H, s, H-8), 6.59 (1H, d, J6,5 9.0,H-6), 7.40 (1H, d, J5,6 9.0, H-5). 13C NMR (100 MHz, CDCl3, , ppm):12.5 (C-11, C-13), 19.2 (C-9), 44.9 (C-10,C-12), 97.3 (C-8), 105.7 (C-3),109.0 (C-4a), 109.1 (C-6), 126.2 (C-5), 150.7 (C-7), 151.6 (C-4), 154.5(C-8a), 158.3 (C-2).
References:
Martins;Candeias;Caldeira;Pereira [Dyes and Pigments,2020,vol. 174,art. no. 108026]

20571-42-0
74 suppliers
$56.00/200mg

92295-93-7
7 suppliers
inquiry