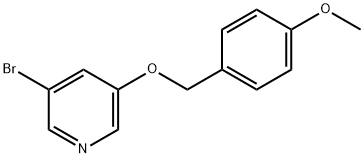
3-Bromo-5-(4-methoxybenzyloxy)pyridine synthesis
- Product Name:3-Bromo-5-(4-methoxybenzyloxy)pyridine
- CAS Number:552331-73-4
- Molecular formula:C13H12BrNO2
- Molecular Weight:294.14

74115-13-2
391 suppliers
$10.00/1g
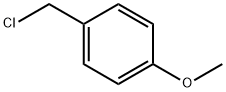
824-94-2
442 suppliers
$24.19/5G

552331-73-4
26 suppliers
$55.00/250mg
Yield:552331-73-4 57%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium carbonate in N,N-dimethyl-formamide at 23; for 84 h;
Steps:
234.A 3-Bromo-5-(4-methoxy-benzyloxy)-pyridine
Example 234A 3-Bromo-5-(4-methoxy-benzyloxy)-pyridine 3-Bromo-5-hydroxypyridine (14.7 g, 84.3 mmol), tetrabutylammonium iodide (0.3 g, 0.8 mmol), and K2CO3 (14.0 g, 101 mmol) were combined in a dry 500 mL round bottom flask with a stirbar. DMF (170 mL) was added followed by PMBCl (12.0 mL, 88.5 mmol). The resulting brown colored mixture was stirred 3.5 days at 23° C. and then silica gel was added and the volatiles removed on a rotary evaporator. Flash chromatography (10-20-40% EtOAc/hexanes) gave 14.1 g (57%) of as an orange solid. Rf=0.52 (50% EtOAc/hexanes) 1H NMR (300 MHz, DMSO-D6) δ ppm 3.32 (s, 3H) 5.12 (s, 2H) 6.96 (m, 2H) 7.39 (m, 2H) 7.79 (m, 1H) 8.28 (d, J=2.03 Hz, 1H) 8.34 (d, J=2.71 Hz, 1H). 13C NMR (100 MHz, DMSO-D6) δ ppm 55.0, 69.8, 113.8, 119.9, 124.0, 127.8, 129.8, 137.1, 142.1, 155.1, 159.2; Anal Calcd for C12H10BrNO: C, 53.08; H, 4.11; N, 4.76. Found: C: 53.00, H: 3.98, N: 4.66.
References:
US2003/199511,2003,A1 Location in patent:Page/Page column 69
625-92-3
479 suppliers
$6.00/10g

552331-73-4
26 suppliers
$55.00/250mg

625-92-3
479 suppliers
$6.00/10g
53942-86-2
0 suppliers
inquiry

552331-73-4
26 suppliers
$55.00/250mg