
3-BROMO-5-MORPHOLINOPYRIDIN-2-AMINE synthesis
- Product Name:3-BROMO-5-MORPHOLINOPYRIDIN-2-AMINE
- CAS Number:1254301-05-7
- Molecular formula:C9H12BrN3O
- Molecular Weight:258.12
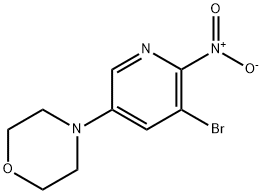
1254301-04-6
0 suppliers
inquiry

1254301-05-7
5 suppliers
inquiry
Yield:1254301-05-7 84%
Reaction Conditions:
with water;tin(ll) chloride in ethanol at 80; for 2 h;Solvent;Reagent/catalyst;Temperature;Concentration;
Steps:
11.6 Step 6. 3-bromo-5-morpholinopyridin-2-amine
Step 6.
3-bromo-5-morpholinopyridin-2-amine
4-(5-Bromo-6-nitropyridin-3-yl)morpholine (95 mg, 0.33 mmol) was dissolved in EtOH (12 mL) and water (3.0 mL) was added, followed by SnCl2 (313 mg, 1.65 mmol).
The reaction mixture was heated to 80° C. for 2 h, cooled to room temperature and diluted with DCM.
The two phases were separated, the organic phase was washed with water.
The water phase was back extracted with DCM.
The pH was adjusted to 12 with 6N NaOH and the mixture was further extracted with DCM. The organic extracts were combined, dried (Na2SO4) and evaporated under reduced pressure obtaining the desired 3-bromo-5-morpholinopyridin-2-amine (71.5 mg, 84%). LCMS (m/z): 260.0 (MH+), 0.37 min.
References:
US9242996,2016,B2 Location in patent:Page/Page column 82; 84
13535-01-8
382 suppliers
$3.00/1g

1254301-05-7
5 suppliers
inquiry

152684-24-7
17 suppliers
inquiry

1254301-05-7
5 suppliers
inquiry
433226-05-2
58 suppliers
inquiry

1254301-05-7
5 suppliers
inquiry
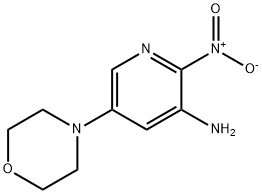
1254300-78-1
1 suppliers
inquiry

1254301-05-7
5 suppliers
inquiry