
3-Bromo-6-nitroindole synthesis
- Product Name:3-Bromo-6-nitroindole
- CAS Number:126807-09-8
- Molecular formula:C8H5BrN2O2
- Molecular Weight:241.04

10097-28-6
162 suppliers
$72.29/50g
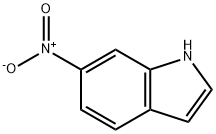
4769-96-4
262 suppliers
$9.00/250mg

126807-09-8
29 suppliers
$201.00/1g
Yield:126807-09-8 88%
Reaction Conditions:
in 2Cl2;
Steps:
11.D.1 1.
1. Preparation of 3-bromo-6-nitroindole N-bromocuccinimide (2.31 g, 12.95 mmol) was added in portions over 5 min to a solution of 6-nitroindole (Lancaster, 2.0 g, 12.33 mmol) in 20 ml dry CH 2Cl2. After 18 hr, the solvent was removed and the solids were chromatographed (200g SiO 2, eluted with 2:1 hexane/ethyl acetate) to give 2.62 g of 3-bromo-6-nitroindole, a yellow solid (88% yield).
References:
EP887346,1998,A2

4769-96-4
262 suppliers
$9.00/250mg

126807-09-8
29 suppliers
$201.00/1g