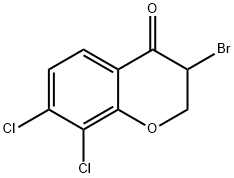
3-BroMo-7,8-dichlorochroMan-4-one synthesis
- Product Name:3-BroMo-7,8-dichlorochroMan-4-one
- CAS Number:1354965-97-1
- Molecular formula:C9H5BrCl2O2
- Molecular Weight:295.94
27407-09-6
13 suppliers
inquiry

1354965-97-1
4 suppliers
inquiry
Yield: 74%
Reaction Conditions:
with pyridinium hydrobromide perbromide in ethanol;chloroform at 50; for 0.5 h;
Steps:
VIII.3.iii
158] Synthesis of 3-bromo-7,8-dichloro-2,3-dihydrochromen-4-one (PC173): Procedure:; A mixture of 100 mg. (0.46 mmol) 7,8-dichlorochroman-4-one was dissolved in anhydrous ethanol (5 ml) and Chloroform (5 ml). To this solution was added pyridinium tribromide (0.442 g, 1.38 mmol, 3 equiv). The reddish brown mixture was heated with stirring at 50 C for 30 min. The reaction mixture was then cooled and the solvent was evaporated. Then water (20 ml) was added to the residue and it was then extracted with 20 ml of dichloromethane. The dichloromethane layer was then washed with 5 % sodium bicarbonate solution followed by water (20 ml). The organic layer was then dried and the solvent was evaporated in vacuum to yield the crude product which was purified by column chromatography.PC173[159] PC173: Pale yellow solid, Yield: 100 mg (74 %); 1H NMR (CDCI3, 400 MHz) in ppm: δ 4.62-4.64 (m, 1H), 4.75-4.80 (m, 1H), 7.20 (d, J= 8.0 Hz, 1H), 7.79 (d, J= 8.0 Hz, 1H); 13C NMR (CDCI3, 100 MHz) in ppm: δ 44.0, 72.2, 1 18.2, 122.2, 123.9, 126.6, 141.5, 157.3, 184.0.
References:
PRESIDENT AND FELLOWS OF HARVARD COLLEGE;CHOREV, Michael;AKTAS, Bertal Huseyin;HALPERIN, José A.;WAGNER, Gerhard WO2012/6068, 2012, A2 Location in patent:Page/Page column 99; 101