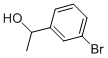
3-BROMO-ALPHA-METHYLBENZYL ALCOHOL synthesis
- Product Name:3-BROMO-ALPHA-METHYLBENZYL ALCOHOL
- CAS Number:52780-14-0
- Molecular formula:C8H9BrO
- Molecular Weight:201.06
3132-99-8
481 suppliers
$5.00/10g
75-24-1
153 suppliers
$54.50/100ml

52780-14-0
93 suppliers
$10.00/1g
Yield: 99%
Reaction Conditions:
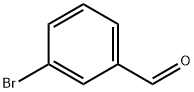
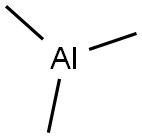
Stage #1:m-bromobenzoic aldehyde;trimethylaluminum in dichloromethane;toluene at -78 - 20; for 1.5 h;
Stage #2: with hydrogenchloride;water in dichloromethane;toluene at 0;
Steps:
A4.A4.1.A4.1a
Example A4; N-[(R,S)1-[3-[7,8-Dimethyl-8H-imidazo[4,5-d]thiazolo[5.4-b]pyridin-2-yl]phenyl]ethyl]acetamide; A4.1 3-(1-Acetamidoethyl)phenylboronic acid; A4.1a: 1-(3-Bromophenyl)ethanol; Commercially available 3-bromobenzaldehyde (5.0 g, 27 mmol) was dissolved in anhydrous dichloromethane (200 mL) under a dry nitrogen atmosphere and cooled in a dry ice/acetone bath to -78° C. Trimethyl aluminum 2M solution in toluene (16.2 mL, 32 mmol) was added dropwise. After 0.5 h the reaction mixture was allowed to warm to room temperature during which time the cloudiness of the reaction resolved to a clear yellow solution which then proceeded to a colorless solution. After stirring for 1 h at room temperature the reaction mixture was cooled in an ice bath and 1N HCl (100 mL) was added dropwise. The reaction mixture was transferred to a separatory funnel and the organic layer was isolated, dried over anhydrous magnesium sulfate, filtered and evaporated under reduced pressure. The crude product was purified by silica gel flash column chromatography eluting with 1:1 Ethyl acetate/hexane to provide 5.4 g (99%) of A4.1a as a colorless oil. LCMS (M+H-OH)+=183, 185
References:
Dyckman, Alaric;Pitts, William J.;Belema, Makonen;Gill, Patrice;Kempson, James;Qiu, Yuping;Quesnelle, Claude;Spergel, Steven H.;Zusi, F. Christopher US2006/106051, 2006, A1 Location in patent:Page/Page column 27-28

2142-63-4
392 suppliers
$5.00/10g

52780-14-0
93 suppliers
$10.00/1g

3132-99-8
481 suppliers
$5.00/10g

75-16-1
265 suppliers
$12.00/10ml

52780-14-0
93 suppliers
$10.00/1g

2142-63-4
392 suppliers
$5.00/10g
67-63-0
1550 suppliers
$16.00/25ML

52780-14-0
93 suppliers
$10.00/1g

67-64-1
6 suppliers
$17.30/10ml
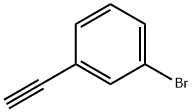
766-81-4
130 suppliers
$12.00/1g

52780-14-0
93 suppliers
$10.00/1g