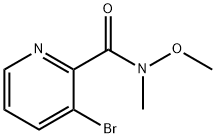
3-Bromo-N-Methoxy-N-Methylpicolinamide synthesis
- Product Name:3-Bromo-N-Methoxy-N-Methylpicolinamide
- CAS Number:867353-49-9
- Molecular formula:C8H9BrN2O2
- Molecular Weight:245.07

6638-79-5
553 suppliers
$6.00/25g
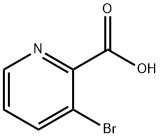
30683-23-9
234 suppliers
$5.00/1g

867353-49-9
17 suppliers
$165.19/100mg
Yield:867353-49-9 78%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate in dichloromethane at 20; for 20 h;
Steps:
439.1 Step 1
3-Bromopicolinic acid (M-64) (2.02 g, 10.0 mmol) was dissolved in DCM (20.0 mL), DIPEA (5.19 mL, 30.0mmol), HATU (4.56 g, 12.0 mmol) and N,O-dimethylhydroxylamine hydrochloride (1.17 g, 12.0 mmol) were added, and the mixture was stirred at room temperature for 20 hr. Water was added to the reaction mixture, and the mixture wasextracted with chloroform. The organic layer was washed successively with water and saturated brine, dried over anhydroussodium sulfate, and filtered. The solvent was evaporated under reduced pressure. The residue was purified bysilica gel column chromatography (NH silica gel, n-hexane:ethyl acetate = 80:20 → 20:80) to give compound (M-65)(yield 1.91 g, 78%) as a white solid
References:
EP3351533,2018,A1 Location in patent:Paragraph 0999; 1000