
(3-BROMOPHENYL)(CYCLOPROPYL)METHANAMINE synthesis
- Product Name:(3-BROMOPHENYL)(CYCLOPROPYL)METHANAMINE
- CAS Number:536694-26-5
- Molecular formula:C10H12BrN
- Molecular Weight:226.11
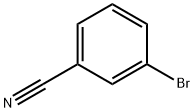
6952-59-6
357 suppliers
$9.00/10g
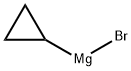
23719-80-4
176 suppliers
$85.20/25ml

536694-26-5
15 suppliers
inquiry
Yield:536694-26-5 10%
Reaction Conditions:
Stage #1: 3-cyanobromobenzene;cyclopropylmagnesium bromide in tetrahydrofuran;methanol at 0 - 50; for 4 h;
Stage #2: with sodium tetrahydroborate in tetrahydrofuran;methanol at 0; for 2.5 h;
Stage #3: with water;ammonium chloride in tetrahydrofuran;methanol;
Steps:
1
Example 1; 1 -(3 -BromophenyD- 1 -cvcloprop ylmethanamine; 3-bromobenzonitrile (2.73 g, 15 mmol) was dissolved in dry tetrahydrofuran (15 mL) and added drop wise to a cyclopropanemagnesiumbromide (36 mL, 18 mmol, 0.5 M in tetrahydrofuran) under an atmosphere of argon. The mixture was heated to 50 °C for 4h and then cooled to 0 °C. Dry methanol (50 mL) was added, the mixture was stirred for 30 min and sodium borohydride (1.14 g, 30 mmol) was added. After 2.5h was saturated aqueous ammonium chloride added and the mixture was extracted with dichloromethane. The organic phases was pooled, dried over magnesium sulfate and concentrated. The residue was suspended in diethyl ether and filtered, the solid was collected and dried in vacuo to give 0.33 g (10% yield) of the title compound: 1H NMR (DMSOd6) δ 7.73 (t, J= 1.88 Hz, 1 H)3 7.56 - 7.52 (m, 1 H), 7.51 - 7.48 (m, 1 H), 7.37 (t, J= 7.91 Hz, 1 H), 3.48 (d, J= 9.54 Hz, 1 H), 1.23 - 1.14 (m, 1 H), 0.66 - 0.59 (m, 1 H), 0.54 - 0.44 (m, 2 H), 0.40 - 0.32 (m, 1 H).
References:
WO2008/63114,2008,A1 Location in patent:Page/Page column 35-36