
propargyl bromide synthesis
- Product Name:propargyl bromide
- CAS Number:106-96-7
- Molecular formula:C3H3Br
- Molecular Weight:118.96
It is derived from the reaction of propargyl alcohol with phosphorus bromide. The propargyl alcohol was added to pyridine under cooling, and phosphorus tribromide was slowly added dropwise with stirring at -5°C, and the reaction temperature was maintained below 0°C. After the addition was completed, stirring was continued for 15 min. Then, vacuum distillation is performed to collect all the distillate, and fractional distillation is carried out again under normal pressure to obtain the finished product. Or add a small amount of pyridine to dry propargyl alcohol, add phosphorus tribromide and a small amount of pyridine solution dropwise at 0 °C under stirring, stir for 20 min after dropping, and distill under reduced pressure to obtain the product. Reaction equation: CH≡C-CH2OH+PBr3→CH≡C-CH2Br

107-19-7
5 suppliers
$12.00/25g

10024-18-7
3 suppliers
inquiry

106-96-7
338 suppliers
$31.00/25g

627-15-6
25 suppliers
$113.00/5g

513-31-5
167 suppliers
$16.00/1g
Yield:106-96-7 78.81% ,10024-18-7 1.38% ,513-31-5 14.78% ,627-15-6 3.17%
Reaction Conditions:
with phosphorus tribromide in toluene at 0 - 50; for 3.25 h;Product distribution / selectivity;
Steps:
4 Example 5
Toluene (50.0 g) and propargyl alcohol (20.0 g, 0.357 mol) were charged to around-bottom flask. Phosphorus tribromide (11.9 mL, 33.9 g, 0.125 mol) was fed to the flask while maintaining the temperature of the contents of the flask at 3 C. During the initial part of the addition, the temperature rose to 15 C. before returning to 3 C. Addition lasted for about 15 minutes, during which this reaction mixture was stirred. The reaction mixture was warmed to 50 C. and allowed to ride, with stirring, for 3 hours at 50 C. The mixture was then washed with water (29.61 g), and stirred for 10 minutes at a rate of 250 rpm. The mixture was then allowed to stand to form aqueous and organic layers; the layers were separated. The organic layer was analyzed by 1HNMR. The yield of propargyl bromide was 78.81%. The solution also contained 14.78% 2,3-dibromopropene, 3.17% 1,3-dibromopropene, and 1.38% bromoallene.
References:
US2004/44259,2004,A1 Location in patent:Page 3

107-19-7
5 suppliers
$12.00/25g

106-96-7
338 suppliers
$31.00/25g

107-19-7
5 suppliers
$12.00/25g

106-96-7
338 suppliers
$31.00/25g

627-15-6
25 suppliers
$113.00/5g

513-31-5
167 suppliers
$16.00/1g

16156-58-4
97 suppliers
$95.00/1g

106-96-7
338 suppliers
$31.00/25g
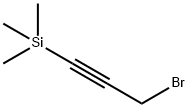
38002-45-8
178 suppliers
$10.00/1g

106-96-7
338 suppliers
$31.00/25g