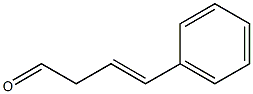
3-Butenal, 4-phenyl- synthesis
- Product Name:3-Butenal, 4-phenyl-
- CAS Number:6005-76-1
- Molecular formula:C10H10O
- Molecular Weight:146.1858
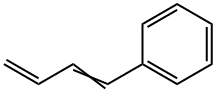
1515-78-2
14 suppliers
inquiry

6005-76-1
0 suppliers
inquiry
Yield:6005-76-1 86%
Reaction Conditions:
with ammonium persulfate;Pd(E-2-(benzylthio)-N-{(2-methoxynaphthalene-1-yl)methylene}benzenamine)Cl;1-butyl-3-methylimidazolium Tetrafluoroborate in water at 70; for 0.25 h;Microwave irradiation;regioselective reaction;Wacker Oxidation;
Steps:
Microwave assisted palladium complex catalyzed selectiveoxidation of terminal olefin to aldehyde
General procedure: To a homogeneous solution of Pd(II) complex (0.5 mol%) in 10 ml[bmim]BF4eH2O (2:1), substituted styrene (1 mmol) was added,followed by ammonium persulfate (1.5 mmol) as oxidant weretaken in glass tubes, sealed and placed in the cavity of microwaveapparatus. Then set parameters are as follows: microwave irradiationpower 80W, increasing time 5 min, target temperature 70 C,standing time 15 min, standing temperature 70 C. A maximumirradiation power of 80Wand 70 C were applied for 15 min. Afterthe temperature reached 70 C, the instrument was automaticallyadjusted to maintain a constant temperature. After completion of the reaction (monitored through TLC), the reaction mixture wascooled to room temperature then diluted with 2 10 ml water,then extracted with ethyl acetate and dried over Na2SO4. Theresulting solution was concentrated under vacuum. Product wasfurther purified by chromatographic separation with a mixture ofethyl acetate:hexane (1:10)
References:
Sarma, Kuladip;Devi, Namita;Sutradhar, Dipankar;Sarma, Bipul;Chandra, Asit K.;Barman, Pranjit [Journal of Organometallic Chemistry,2016,vol. 822,p. 20 - 28]
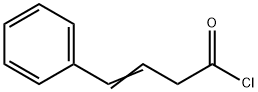
60773-92-4
0 suppliers
inquiry

6005-76-1
0 suppliers
inquiry
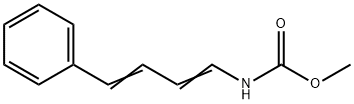
53106-73-3
0 suppliers
inquiry

6005-76-1
0 suppliers
inquiry