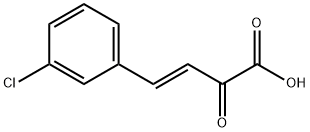
3-Butenoic acid, 4-(3-chlorophenyl)-2-oxo-, (3E)- synthesis
- Product Name:3-Butenoic acid, 4-(3-chlorophenyl)-2-oxo-, (3E)-
- CAS Number:1244025-00-0
- Molecular formula:C10H7ClO3
- Molecular Weight:210.61
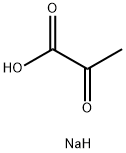
Yield:1244025-00-0 95 %
Reaction Conditions:
with NahE in aq. phosphate buffer;dimethyl sulfoxide at 20;Enzymatic reaction;
Steps:
Enzymatic Synthesis of α,β-unsaturated 2-keto acids (General ProcedureB)
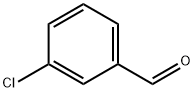
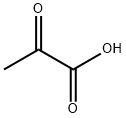
General procedure: Aldehyde (1 mmol) was added to 100 mM potassium phosphate buffer(pH 6.5, 20 mL) containing sodium pyruvate (357 mg, 3.24 mmol) andDMSO (2 mL, ≤ 10 % v/v). NahE (7.9 mg, 0.02 mol%) was added to initiatethe reaction and mixed at room temperature for 16 h. Any residualaldehyde was extracted with ethyl acetate (2 × 20 mL) and the aqueousphase was then acidified to pH 2 and extracted with ethyl acetate (3 × 20mL). The organic layer was washed with water (3 × 30 mL), brine (2 × 30mL) and dried over sodium sulfate. The solvent was removed undervacuum to give corresponding substituted α, β-unsaturated 2-keto acids.All reactions were performed in duplicate with the average mass and yieldreported. No further purification was required. Isolated products werehomogeneous as assessed by HPLC and/or 1H NMR.
References:
Fansher, Douglas J.;Ngwira, Niza;Salehi, Ahmad Reza;Woods, Jerome;Casc?o, Amanda;Palmer, David R. J. [Synthesis,2022,vol. 55,# 1,p. 75 - 89]