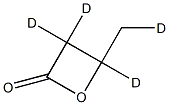
3-Butyrolactone-d4 synthesis
- Product Name:3-Butyrolactone-d4
- CAS Number:1224441-94-4
- Molecular formula:C4H2D4O2
- Molecular Weight:90.11388

77568-65-1
37 suppliers
$496.00/50mg

1224441-94-4
4 suppliers
inquiry
Yield:1224441-94-4 94%
Reaction Conditions:
Stage #1: <2H6>γ-Butyrolactonewith sodium in methanol; for 32 h;Reflux;
Stage #2: with hydrogenchloride;acetic acid in methanol;water at 20;
Steps:
9.1
Sodium metal (2.1 g, 90.6 mmol, 0.56 eq) was dissolved in MeOH (180 mL). To this was added γ- butyrolactone-ck (38-rfβ) (15.0 g, 163 mmol, 1 eq; Aldrich, 98 atom% D) as a solution in MeOH (180 mL). The resulting solution was heated at reflux for 16 hours. The reaction was cooled to room temperature and concentrated under reduced pressure. A fresh portion of MeOH (360 mL) was added to the residue and the reaction was heated at reflux for an additional 16 hours. The reaction was cooled to room temperature and quenched by the addition of acetic acid (5.3 mL, 90.0 mmol) and several drops of concentrated HCl. The solvent was evaporated under reduced pressure and CH2Cl2 (100 mL) was added. The resulting suspension was filtered and the filtrate was evaporated under reduced pressure leaving a slightly yellow oil (γ- hydroxy-methylbutyrate-d*). This material was dissolved in H2O (100 mL) and concentrated HCl (9 mL) was added. The reaction was heated at reflux for 1 hour and then cooled to room temperature. The solution was saturated with NaCl and the product extracted with CH2Cl2 (3 x 500 mL). The combined organics were dried over Na2SO4, filtered, and evaporated to give 15.1 g of a yellow oil. The crude product was purified by Kugelrohr distillation yielding 13.9 g (94%) of 38-4 as a colorless liquid.
References:
WO2010/47819,2010,A1 Location in patent:Page/Page column 53-54