
3-CHLORO-1,1,1,3-TETRAFLUOROPROPANE synthesis
- Product Name:3-CHLORO-1,1,1,3-TETRAFLUOROPROPANE
- CAS Number:149329-29-3
- Molecular formula:C3H3ClF4
- Molecular Weight:150.5

15022-22-7
0 suppliers
inquiry
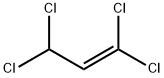
18611-43-3
13 suppliers
inquiry

149329-29-3
14 suppliers
inquiry
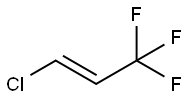
102687-65-0
38 suppliers
inquiry
Yield:102687-65-0 86.0 %Chromat. ,149329-29-3 5.5 %Chromat.
Reaction Conditions:
with hydrogen fluoride;titanium tetrachloride at 85; under 6965.99 Torr;
Steps:
3 Example 3
This example (called Run 3) illustrates the semi-batch reaction where HF is continuously fed into a charge of titanium tetrachloride catalyst and a mixture of 1,1,1,3-tetrachloropropene and 1,1,3,3-tetrachloropropene. [0086] A clean, empty 10-gallon jacketed, agitated reactor of Hastelloy C construction is prepared. This reactor is connected to a 2 inch diameter vertical, PTFE-lined pipe containing packing material (stripper), which is in turn connected to an overhead heat exchanger. The heat exchanger is supplied with -40° C. brine circulation on the shell side. Vapors exiting this stripper are processed through a scrubber, in which temperature-controlled dilute potassium hydroxide aqueous solution is circulated. Vapors exiting this stripper are collected in a weighed, chilled (-40° C.) cylinder referred to as the product collection cylinder (PCC), followed by a smaller cylinder in series chilled in a dry ice bath. [0087] For the experiment, 14 lbs of anhydrous HF is charged to the reactor for the purpose of fluorinating TiCl4 catalyst plus a small excess to start the reaction. Next, 1.5 lbs of TiCl4 is added as a catalyst. HCl is immediately generated as observed by the build-up of pressure in the reactor. After the pressure is reduced by venting most of the HCl from the system, 50 lbs of a mixture of 1,1,1,3-tetrachloropropene and 1,1,3,3-tetrachloropropene is added. The reactor is then heated by applying steam to the reactor jacket. At about 85° C. HCl started to be generated indicating that the fluorination reaction is initiated. The system pressure is controlled at about 120 psig. Additional HF is then fed continuously and product is collected in the product collection cylinder until the majority of the mixture of 1,1,1,3-tetrachloropropene and 1,1,3,3-tetrachloropropene is consumed. The GC analysis of the crude material collected during the run is as follows: 86.0% 1233zd (E); 5.5% 244fa; 3.0% 1234ze (E); 1.5% 1233zd (Z); 1.1% 1234ze (Z); 0.3% 245fa; 0.5% C6 compounds (dimers); and 0.2% trifluoropropyne.
References:
US2013/261354,2013,A1 Location in patent:Paragraph 0085; 0086; 0087

23153-23-3
13 suppliers
$45.00/5G

149329-29-3
14 suppliers
inquiry

460-69-5
22 suppliers
inquiry
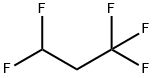
460-73-1
1 suppliers
inquiry
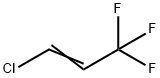
2730-43-0
40 suppliers
inquiry

23153-23-3
13 suppliers
$45.00/5G

149329-29-3
14 suppliers
inquiry
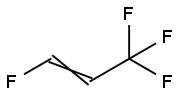
1645-83-6
34 suppliers
inquiry

460-73-1
1 suppliers
inquiry

2730-43-0
40 suppliers
inquiry